Publications
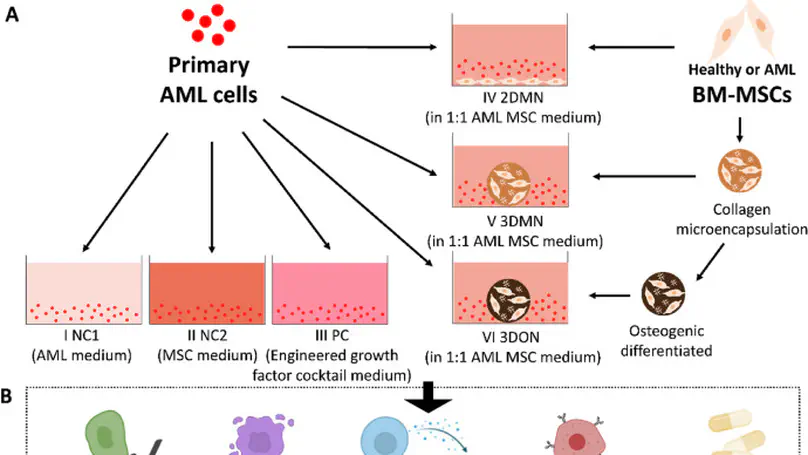
Acute myeloid leukemia (AML) is a deadly form of leukemia with ineffective traditional treatment and frequent chemoresistance-associated relapse. Personalized drug screening holds promise in identifying optimal regimen, nevertheless, primary AML cells undergo spontaneous apoptosis during cultures, invalidating the drug screening results. Here, we reconstitute a 3D osteogenic niche (3DON) mimicking that in bone marrow to support primary AML cell survival and phenotype maintenance in cultures. Specifically, 3DON derived from osteogenically differentiated mesenchymal stem cells (MSC) from healthy and AML donors are co-cultured with primary AML cells. The AML cells under the AML_3DON niche showed enhanced viability, reduced apoptosis and maintained CD33+ CD34-phenotype, associating with elevated secretion of anti-apoptotic cytokines in the AML_3DON niche. Moreover, AML cells under the AML_3DON niche exhibited low sensitivity to two FDA-approved chemotherapeutic drugs, further suggesting the physiological resemblance of the AML_3DON niche. Most interestingly, AML cells co-cultured with the healthy_3DON niche are highly sensitive to the same sample drugs. This study demonstrates the differential responses of AML cells towards leukemic and healthy bone marrow niches, suggesting the impact of native cancer cell niche in drug screening, and the potential of re-engineering healthy bone marrow niche in AML patients as chemotherapeutic adjuvants overcoming chemoresistance, respectively.
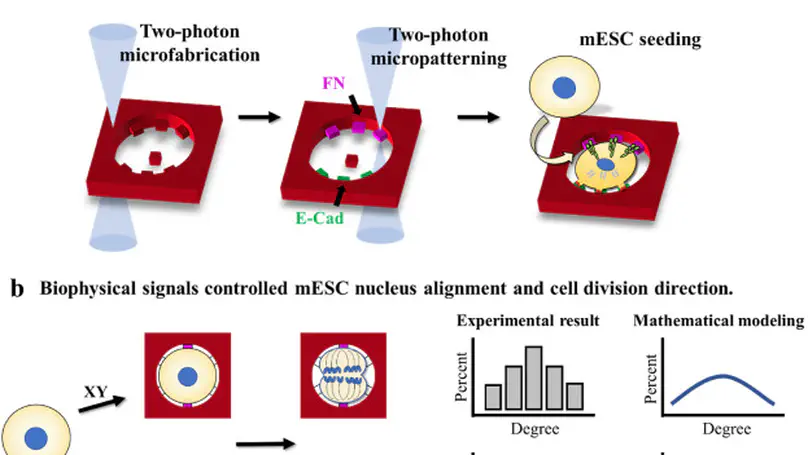
Intricate microenvironment signals orchestrate to affect cell behavior and fate during tissue morphogenesis. However, the underlying mechanisms on how specific local niche signals influence cell behavior and fate are not fully understood, owing to the lack of in vitro platform able to precisely, quantitatively, spatially, and independently manipulate individual niche signals. Here, microarrays of protein-based 3D single cell micro-niche (3D-SCµN), with precisely engineered biophysical and biochemical niche signals, are micro-printed by a multiphoton microfabrication and micropatterning technology. Mouse embryonic stem cell (mESC) is used as the model cell to study how local niche signals affect stem cell behavior and fate. By precisely engineering the internal microstructures of the 3D SCµNs, we demonstrate that the cell division direction can be controlled by the biophysical niche signals, in a cell shape-independent manner. After confining the cell division direction to a dominating axis, single mESCs are exposed to asymmetric biochemical niche signals, specifically, cell-cell adhesion molecule on one side and extracellular matrix on the other side. We demonstrate that, symmetry-breaking (asymmetric) niche signals successfully trigger cell polarity formation and bias the orientation of asymmetric cell division, the mitosis process resulting in two daughter cells with differential fates, in mESCs.
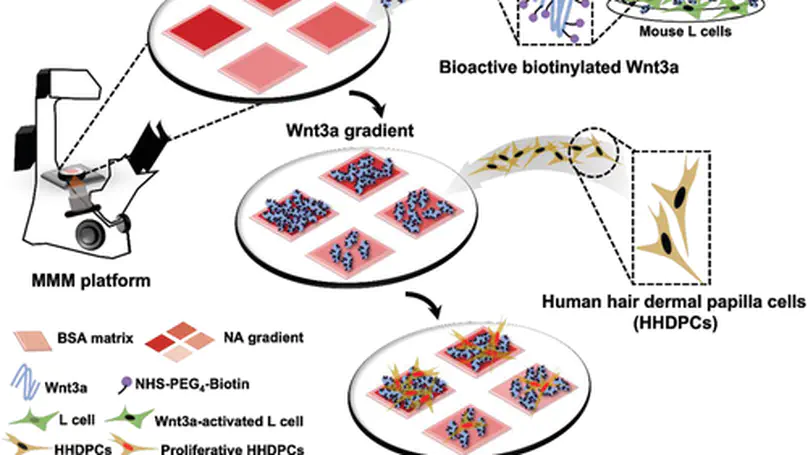
The Wingless/Integrated (Wnt) signaling is a central player in regulating multiple cellular events ranging from developmental process, adult homeostasis to tumorigenesis. In native microenvironments, Wnt signals often function through the concentration gradients between the secreting site and the targeting site. Hence, presenting Wnt molecules in a concentration gradient in vitro in a controllable manner recapitulates the native Wnt niche. Nevertheless, most in vitro Wnt niche models, where the ligands are homogeneously immobilized on material surface, fail in reconstituting the concentration gradient of Wnt as that in vivo. Herein, using a previously established Multiphoton Microfabrication and Micropatterning (MMM) technology, a concentration gradient of Wnt3a is immobilized on a protein substrate via neutravidin-biotin interactions, in a quantitatively and spatially controllable manner. Such Wnt3a gradient is bioactive as it triggers a dose- and time-dependent upregulation of β-catenin and cell proliferation, in model mouse L cells. Most importantly, human hair dermal papilla cells (HHDPCs) cultured in a cell niche with Wnt3a gradient proliferate in a Wnt3a-gradient-dependent manner and exhibit a Wnt3a-gradient-dependent hair-inductive phenotype including Wnt/β-catenin signaling activation and alkaline phosphatase (ALP) expression. This work contributes to the future development of a hair follicle inductivity biochip for screening of drugs and therapeutics on hair regeneration.
The nucleus pulposus (NP) of intervertebral disc represents a soft gel consisting of glycosaminoglycans (GAGs)-rich extracellular matrix (ECM). Significant loss of GAGs and normal functions are the most prevalent changes in degenerated disc. Attempts targeted to incorporate GAGs into collagen fibrous matrices have been made but the efficiency is very low, and the resulting structures showed no similarity with native NP. Inspired by the characteristic composition and structures of the ECM of native NP, here, we hypothesize that by chemically modifying the collagen (Col) and hyaluronic acid (HA) and co-precipitating with GAGs, a bio-inspired nano-material recapitulating the composition, ultra-structure and function of the GAG-rich ECM will be fabricated. Compositionally, the bio-inspired nano-material namely Aminated Collagen-Aminated Hyaluronic Acid-GAG (aCol-aHA-GAG) shows a record high GAG/hydroxyproline ratio up to 39.1:1 in a controllable manner, out-performing that of the native NP. Ultra-structurally, the nano-material recapitulates the characteristic ’nano-beads’ (25~nm) and ‘bottle-brushes’ (133~nm) features as those found in native NP. Functionally, the nano-material supports the viability and maintains the morphological and phenotypic markers of bovine NP cells, and shows comparable mechanical properties of native NP. This work contributes to the development of a compositionally, structurally, and functionally biomimetic nano-material for NP tissue engineering.
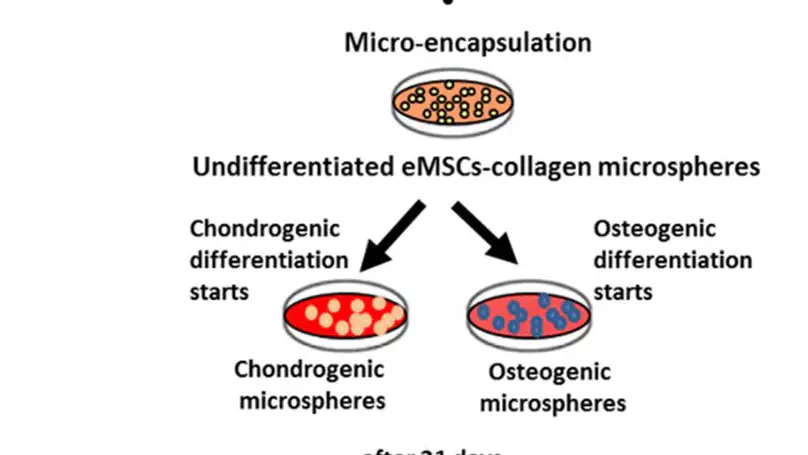
Damage to the hyaline cartilage of the joint surface and osteochondral fractures are key factors leading to the development of osteoarthritis in racehorses, representing a significant cause of racehorse retirement. To tissue-engineer an osteochondral unit that is suitable for joint repair, incorporation of a zone of calcified cartilage should be considered so as to mimic its in vivo counterpart. To date, equine mesenchymal stem cells (eMSCs) have been reported to have multilineage differentiation potential. Yet the generation of a zone of calcified cartilage using eMSCs has not been reported. This work is an initial attempt to generate a zone of calcified cartilage using eMSCs as the single source of cells and collagen as the scaffolding material. Main advantages of using eMSCs over equine deep zone chondrocytes for the generation of a zone of calcified cartilage include no donor site morbidity and their ease of expansion in culture. Initially, we fabricated cartilage-like tissues and bone-like tissues in vitro by differentiating eMSCs toward chondrogenic and osteogenic lineages for 21 d, respectively. We then aggregated the cartilage-like and bone-like tissues together with a layer of undifferentiated eMSCs-collagen gel in between to generate a 3-layer osteochondral unit. A zone of calcified cartilage was found between the cartilage-like and bone-like layers after a 14-day culture in chondrogenic differentiation medium. These results provide a solution toward tissue engineering of equine osteochondral units with interfacial zone without using chondrocytes harvested from the deep zone of healthy articular cartilage, and contribute to the future development of osteochondral tissue engineering strategies for human cartilage injuries in the long run.
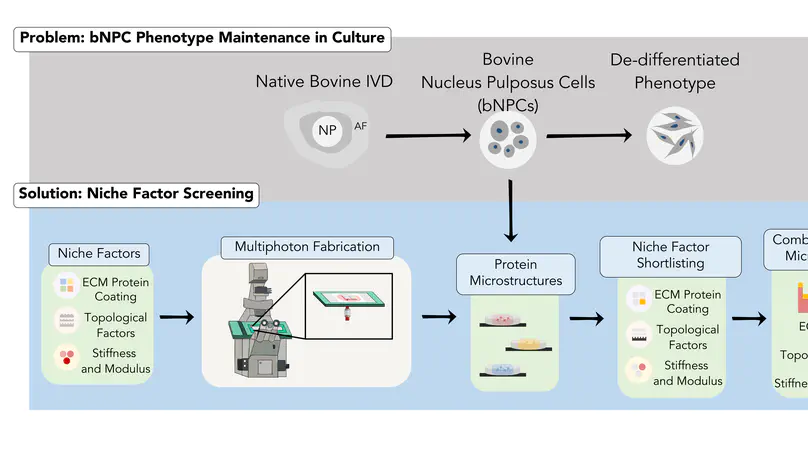
Upon monolayer cultures on flat and rigid plastic dishes, many cells de-differentiate and lose their native phenotype. Technologies able to identify and reconstitute the cell niche factors that best maintain the physiological cellular phenotype in cultures are critical. We have developed a multiphoton microfabrication and micropatterning (MMM) technology, a robust 3D micro-printing platform capable to fabricate protein microstructures and micropatterns with quantitative, spatial and independent control of the mechanical, topological and extracellular matrix properties. Here, using bovine nucleus pulposus cells (bNPCs) as an example, we aim to reconstitute a spectrum of individual cell niche factors (2 mechanical, 9 topological and 4 matrices) in vitro for multiplex cell niche factor screening, and fabricate the optimal combinations of a series of shortlisted cell niche factors that best maintain the bNPC phenotype. Among all factors screened, two topological (micropillar array; fiber-bead structure) and two matrix (laminin; vitronectin) factors were shortlisted and the combinatory cell niche factors reconstituted from the shortlisted factors were found to synergistically augmented the expression of selected bNPC phenotype markers (Col II, SNAP25 and Keratin 8) and maintained their morphology and phenotype. These optimal cell niches can be micro-printed on culture dishes for physiologically relevant cultures and contribute to biomimetic scaffold design for intervertebral disc tissue engineering.
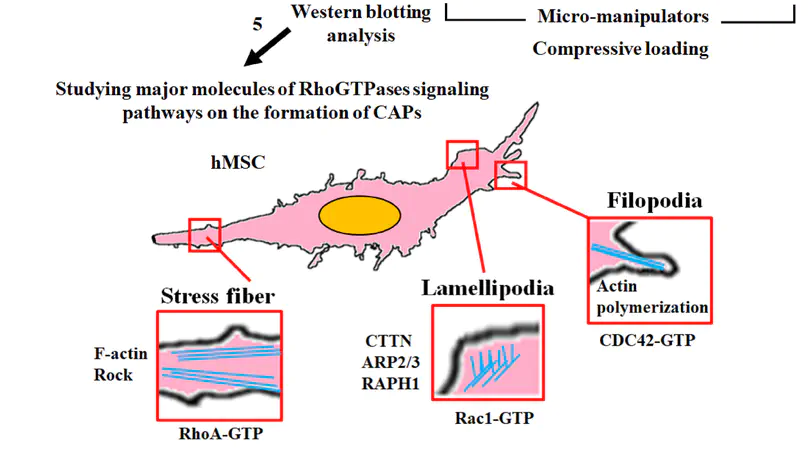
Cells can sense mechanical signals through cytoskeleton reorganization. We previously discovered the formation of omni-directional actin protrusions upon compression loading, namely compression-induced actin protrusions (CAPs), in human mesenchymal stem cells (MSCs) in 3D micro-tissues. Here, the regulatory roles of three RhoGTPases (CDC42, Rac1 and RhoA) in the formation of CAPs were investigated. Upon compression loading, extensive formation of CAPs was found, significantly associated with an upregulated mRNA expression of Rac1 only, but not CDC42, nor RhoA. Upon chemical inhibition of these RhoGTPase activity during compression, only Rac1 activity was significantly suppressed, associating with the reduced CAP formation. Silencing the upstream regulators of these RhoGTPase pathways including Rac1 by specific siRNA dramatically disrupted actin cytoskeleton, distorted cell morphology and aborted CAP formation. Silencing cortactin (CTTN), a downstream effector of the Rac1 pathway, induced a compensatory upregulation of Rac1, enabling the MSCs to respond to the compression loading stimulus in terms of CAP formation, although at a reduced number. The importance of Rac1 signalling in CAP formation and the corresponding upregulation of lamellipodial markers also suggest that these CAPs are lamellipodia in nature. This study delineates the mechanism of compression-induced cytoskeleton reorganization, contributing to rationalizing mechanical loading regimes for functional tissue engineering.
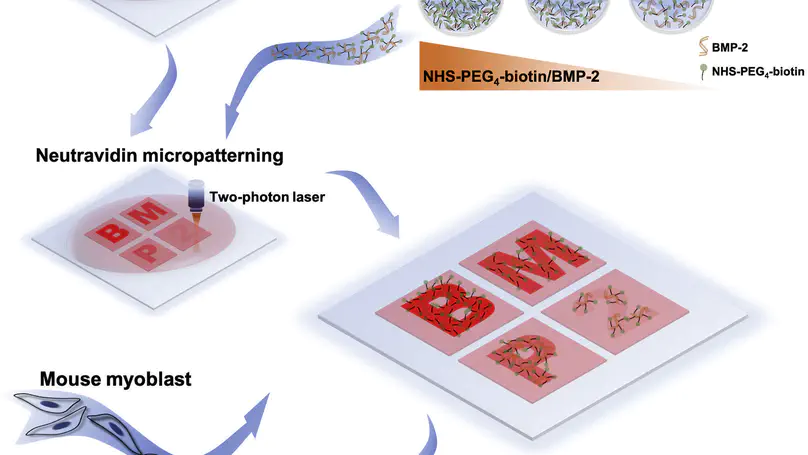
Engineered biomimetic cell niches represent a valuable in vitro tool for investigating physiological and pathological cellular activities, while developing an all-in-one technology to engineer cell niches, particularly soluble cell niche factors, with retained bioactivities, remains challenging. Here, we report a mask-free, non-contact and biocompatible multiphoton microfabrication and micropatterning (MMM) technology in engineering a spatially and quantitatively controllable bone morphogenetic protein-2 (BMP-2) soluble niche, by immobilizing optimally biotinylated BMP-2 (bBMP-2) on micro-printed neutravidin (NA) micropatterns. Notably, the micropatterned NA bound-bBMP-2 niche elicited a more sustained and a higher level of the downstream Smad signaling than that by free BMP-2, in C2C12 cells, suggesting the advantages of immobilizing soluble niche factors on engineered micropatterns or scaffold materials. This work reports a universal all-in-one cell niche engineering platform and contributes to reconstituting heterogeneous native soluble cell niches for signal transduction modeling and drug screening studies.
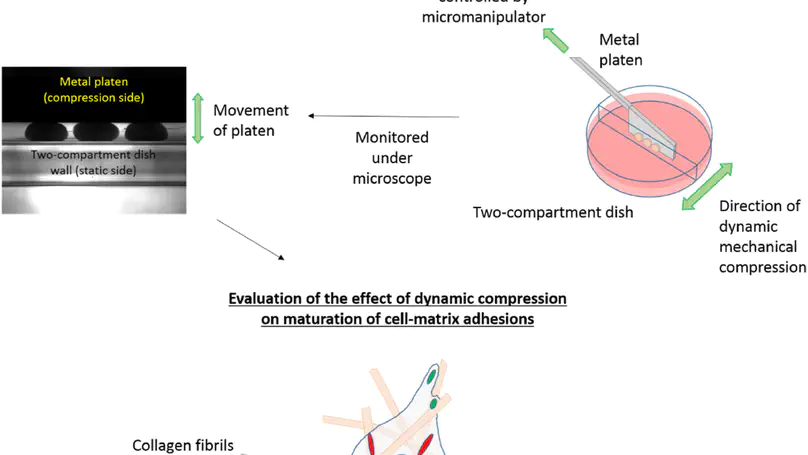
Mechanical signal is important for regulating stem cell fate, but the molecular mechanisms involved are unclear. Cell-matrix adhesions are important molecular mechanosensors that their formation and maturation are force-dependent processes. However, most studies focused on the role of cell contractility or substrate stiffness in these processes. How external mechanical force stimulates the formation and maturation of cell-matrix adhesions is largely unknown. Here, by using human mesenchymal stem cells (hMSCs)-collagen microtissues as a 3D model, we found that upon short-term dynamic compression, integrin $α$V binding, focal adhesion formation, and subsequent FAK activation, are stimulated. This compression-stimulated FAK signaling also leads to YAP activation, suggesting crosstalk between integrin-based signaling and mechanosensing. More importantly, long-term compression induces maturation of $α$5-integrin based adhesions to form long, slender 3D-matrix adhesions (3DMAs), which are distinct from 2D focal adhesions in composition and morphology and previously found only in cell-derived matrices and native tissues. Mechanical preconditioning hMSCs with long-term compression loading induces the formation of mature integrin $α$5-dependent 3DMAs and potentiates their osteogenesis. Collectively, this work shows that active mechanical stimulation can modulate cell-matrix interactions significantly at the cell-material interfaces in a dynamic manner, and affects cell fate decisions, demonstrating the significance of loading-based functional tissue engineering.
Extracellular matrix (ECM) provides both physical support and bioactive signals such as growth factors and cytokines to cells at their microenvironment or niche. Engineering the matrix niche becomes an important approach to study or manipulate cellular fate. This work presents an overview on the reconstitution of the ECM niche through a wide range of approaches ranging from coating culture dish with ECM molecules to decellularization of native tissues. In particular, we focused on reconstituting the complex ECM niche through cell-derived matrix (CDM) by reviewing the methodological approaches used in our group to derive ECM from mature cells such as chondrocytes and nucleus pulposus cells (NPCs), undifferentiated stem cells such as mesenchymal stem cells (MSCs), as well as MSCs undergoing chondrogenic and osteogenic differentiation, in 2D or 3D models. Specific attention has also been given to key factors that should be considered in various applications and challenges in relation to the CDM. Last but not the least, a few future perspectives and their significance have been proposed.
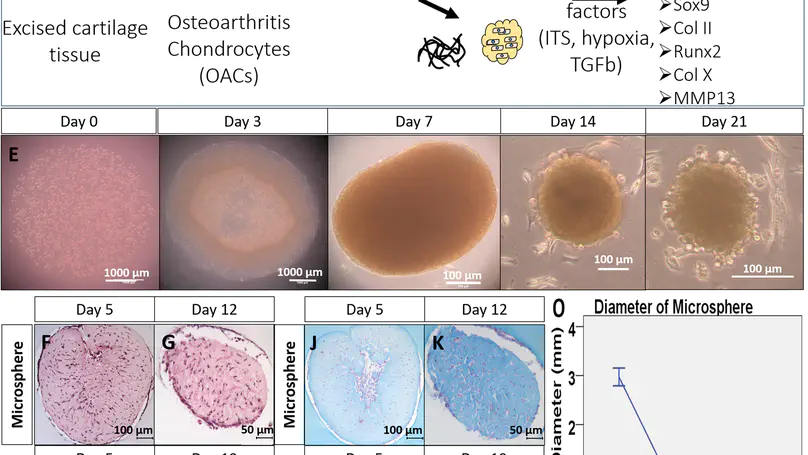
The current study aims to evaluate collagen microencapsulation as an in vitro 3D culture platform for human osteoarthritic chondrocytes (hOACs), and to exemplify its feasibility in screening potential disease modifying factors. We first isolated and expanded hOACs from osteoarthritis (OA) cartilage samples harvested from multiple patients during total knee replacement (TKR) surgery. These cells were microencapsulated into collagen microspheres for subsequent 3D cultures. The change in chondrocyte phenotypes and OA phenotype was evaluated over time, using 2D monolayer culture and traditional 3D pellet culture as controls. The hOACs in the 3D collagen microsphere model resumed their in vivo phenotypes when compared to 2D monolayer. When compared with the 3D pellet model, the 3D hOAC-collagen microsphere model better recapitulated the OA phenotypes. We further demonstrated the responsiveness of the microencapsulated hOACs towards a number of external factors altering the chondrogenic phenotype, corroborating with previous studies. The hOAC encapsulated collagen microspheres better maintained the hOAC phenotype than the traditional 2D monolayer and 3D pellet cultures. The feasibility to use this hOAC-collagen microsphere in vitro model as a screening platform for disease-modifying agents has been demonstrated, contributing to future development of OA therapeutics.
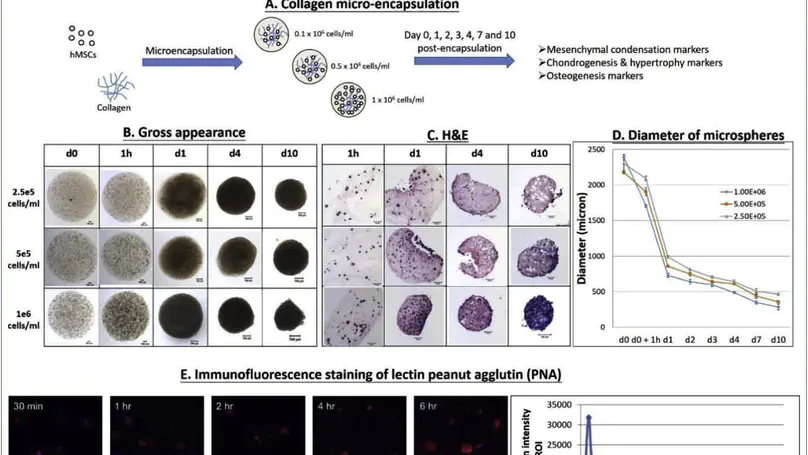
Mesenchymal condensation is a critical transitional stage that precedes cartilage or bone formation. A microencapsulation technique was previously established to entrap mesenchymal stem cells (MSC) in nanofibrous collagen meshwork. We hypothesize that collagen microencapsulation of MSCs mimics the mesenchymal cell condensation process. Specifically, human MSCs at different concentrations were microencapsulated in collagen for different time points before evaluation for early skeletogenesis markers. A transient upregulation of mesenchymal condensation markers including peanut agglutinin, fibronectin, integrins α5 and αv, an enhanced nuclear localization of SOX9 and binding interactions with COL2A1, and other changes in chondrogenic, hypertropic and osteogenic marker were demonstrated. Collagen microencapsulation upregulated both the chondrogenic and the osteogenic transcription factors and the encapsulated hMSCs hold the potential to differentiate towards both chondrogenic and osteogenic lineages. We also hypothesize that collagen microencapsulation potentiates MSC chondrogenesis. Particularly, chondrogenic differentiation of hMSCs were induced at different time post-encapsulation before evaluation for chondrogenesis outcomes. Sustained SOX9, ACAN and COL2A1 expression were noted and the timing to induce supplement chondro-inductive factors matters. This study reports an extracellular matrix-based in vitro model of mesenchymal condensation, an early stage in skeletogenesis, contributing to rationalizing development-inspired tissue engineering.
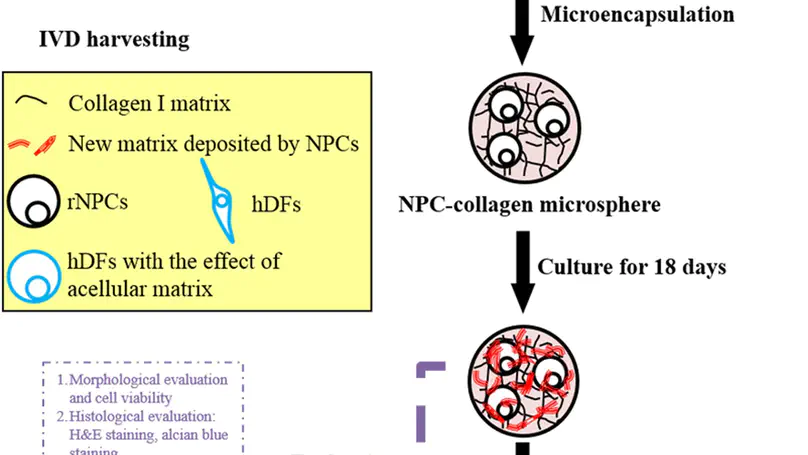
Reconstituting biomimetic matrix niche in vitro and culturing cells at the cell niche interface is necessary to understand the effect and function of the specific matrix niche. Here we attempted to reconstitute a biomimetic extracellular matrix (ECM) niche by culturing nucleus pulposus cells (NPCs) in a collagen microsphere system previously established and allowing them to remodel the template matrix. The reconstituted NPC-derived complex ECM was obtained after decellularization and the composition of such niche was evaluated by proteomic analysis. Results showed that a complex acellular matrix niche consisting of ECM proteins and cytoskeletal proteins by comparing with the template collagen matrix starting material. In order to study the significance of the NPC-derived matrix niche, dermal fibroblasts were repopulated in such niche and the phenotypes of these cells were changed, gene expression of collagen type II and CA12 increased significantly. A biomimetic NPC-derived cell niche consisting of complex ECM can be reconstituted in vitro, and repopulating such matrix niche with fibroblasts resulted in changes in phenotypic markers. This work reports a 3D in vitro model to study cell niche factors, contributing to future understanding of cellular interactions at the cell-niche interface and rationalized scaffold design for tissue engineering.
Reconstituting the biomimetic cell niche in vitro is important as it allows to determine how niche factors may affect cellular fate processes. Many biofabrication and micropatterning technologies are developed to achieve this goal. However, the critical outstanding challenges are the capabilities to spatially control the micropatterns and to decouple the different niche factors. Multiphoton microfabrication is an emerging technology with unique advantages. A multiphoton 3D microprinting platform has been previously established to create protein microstructures and micropatterns with sub-micrometer resolution and its ability to control the topological features, porosity, and mechanical properties of microprinted protein cell niche has been demonstrated. Here, it is demonstrated that the multiphoton microprinting technology is able to precisely control the local density of the matrix niche factor, and more importantly, spatially control and decouple the mechanical and matrix niche factors. The results show that 1) the micropatterned matrix niche retains its bioactivities; 2) mesenchymal stem cells (MSCs) are able to sense the elastic modulus of the cell niche when the mechanical and matrix niche factors are decoupled; and 3) MSCs prefer to bind to and spread on fibronectin among other matrix proteins. These results path the way to future development of a “programmable” cell niche platform.
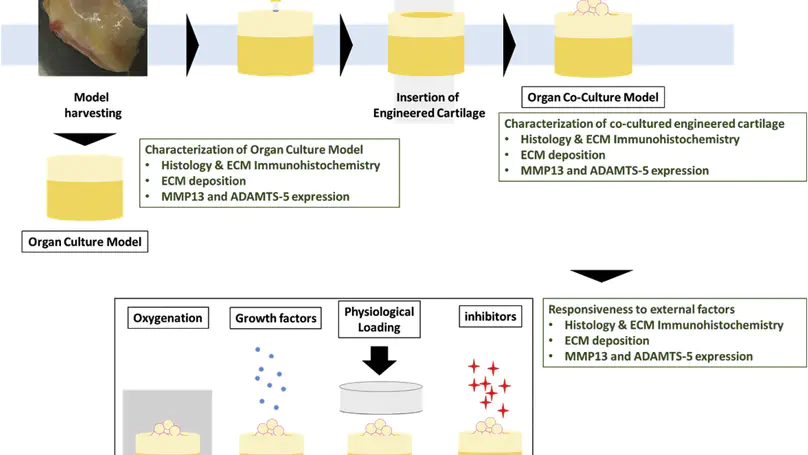
In vitro human osteoarthritis (OA)-mimicking models enabling pathophysiological studies and evaluation of emerging therapies such as cartilage tissue engineering are of great importance. We describe the development and characterization of a human OA osteochondral organ culture. We also apply this model for evaluation of the phenotype maintenance of a human MSC derived engineered cartilage, as an example of emerging therapeutics, under long term exposure to the OA-mimicking environment. We also test the sensitivity of the model to a series of external factors and a potential disease-modifying agent, in terms of chondrogenic phenotype maintenance of the engineered cartilage, under OA-mimicking environment. Excised joint tissues from total knee replacement surgeries were carved into numerous miniaturized and standardized osteochondral plugs for subsequent OA organ culture. The organ cultures were characterized in detail before being co-cultured with a tissue engineered cartilage. The chondrogenic phenotype of the tissue engineered cartilage co-cultured in long term up to 8 weeks under this OA-mimicking microenvironment was evaluated. Using the same co-culture model, we also screened for a number of biomimetic environmental factors, including oxygen tension, the presence of serum and the application of compression loading. Finally, we studied the effect of a matrix metalloprotease inhibitor, as an example of potential disease-modifying agents, on the co-cultured engineered cartilage. We demonstrate that cells in the OA organ culture were viable while both the typical chondrogenic phenotype and the characteristic OA phenotype were maintained for long period of time. We then demonstrate that upon co-culture with the OA-mimicking organ culture, the engineered cartilage initially exhibited a more fibrocartilage phenotype but progressively reverted back to the chondrogenic phenotype upon long term co-culture up to 8 weeks. The engineered cartilage was also found to be sensitive to all biomimetic environmental factors screened (oxygen tension, serum and compression). Moreover, under the effect of a MMP inhibitor, the chondrogenic phenotype of engineered cartilage was better maintained. We demonstrated the development of a human OA osteochondral organ culture and tested the feasibility and potential of using this model as an in vitro evaluation tool for emerging cartilage therapies.
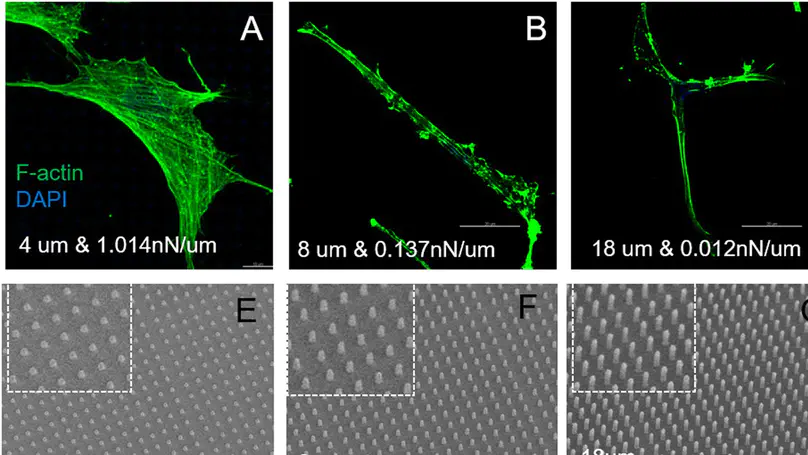
While cells are known to sense and respond to their niche including the matrix and the mechanical microenvironment, whether they preferentially sense and react to the stiffness of their microenvironment regardless of its intrinsic material properties is unknown. In this work, protein micropillar arrays with independently controllable stiffness via alterations in pillar height and elastic modulus via laser power used during photochemical cross-linking, were fabricated using a recently developed multiphoton-based 3D protein micro-patterning technology. Human dermal fibroblasts were cultured on these micropillar arrays and the specific interactions between cells and the protein micropatterns particularly on the formation and maturation of the cell-matrix adhesions, were investigated via immunofluorescence staining of the major molecular markers of the adhesions and the measurement of their cluster size, respectively. Our results showed that the cluster size of focal adhesions increased as the stiffness of the micropillar arrays increased, but it was insensitive to the elastic modulus of the protein micropillars that is one of the intrinsic material properties. This finding provides evidence to the notion that cells preferentially sense and react to the stiffness, but not the elastic modulus of their microenvironment.
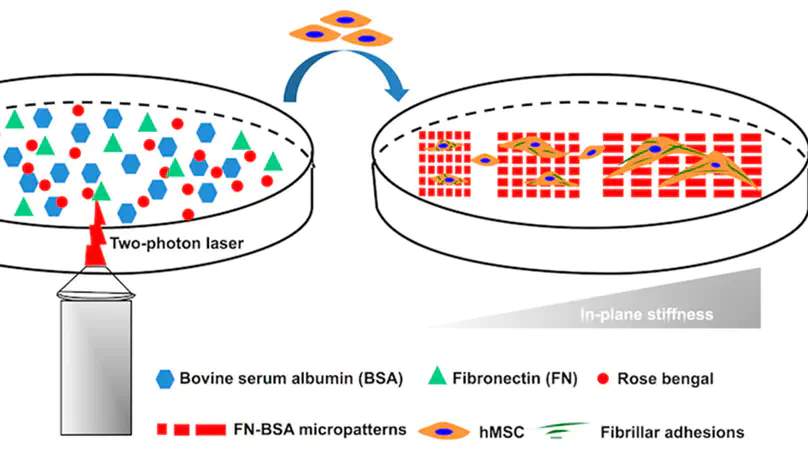
Cell– matrix adhesions are important structures governing the interactions between cells and their microenvironment at the cell– matrix interface. The focal complex (FC) and focal adhesion (FA) have been substantially investigated in conventional planar culture systems using fibroblasts as an in vitro model. However, the formation of more mature types of cell– matrix adhesion in human mesenchymal stem cells (hMSCs), including fibrillar adhesion (FBA) and 3D matrix adhesion (3DMA), have not been fully elucidated. Here we investigate the niche factor(s) that influence(s) the maturation of FBA and 3DMA by using multiphoton fabrication-based micropatterning. First, the bovine serum albumin (BSA)-made protein micropatterns were functionalized by incorporating various concentrations of fibronectin (FN) in fabrication solution. The amount of cross-linked FN is positively correlated with the initial concentration of FN in the reaction liquid, as verified by immunofluorescence staining. On the other hand, the anisotropic FN-functionalized micropatterns were fabricated by varying the length (i.e., in-plane stiffness) and height (i.e., bending stiffness) of micropatterns, respectively. Finally, hMSCs were cultured on these micropatterns for 2 h and 1 day to determine the formation of FBA and 3DMA, respectively, using immunofluorescence staining. Results demonstrated that FN-functionalized micropatterns with high anisotropy in x– y dimension benefit FBA maturation. Furthermore, niche factors such as higher bending and in-plane stiffness and the presence of abundant fibronectin have a positive effect on the maturation of FN-based cell– matrix adhesion. These findings could provide some new perspectives on designing platforms for further cell niche study and rationalizing scaffold design for tissue engineering.
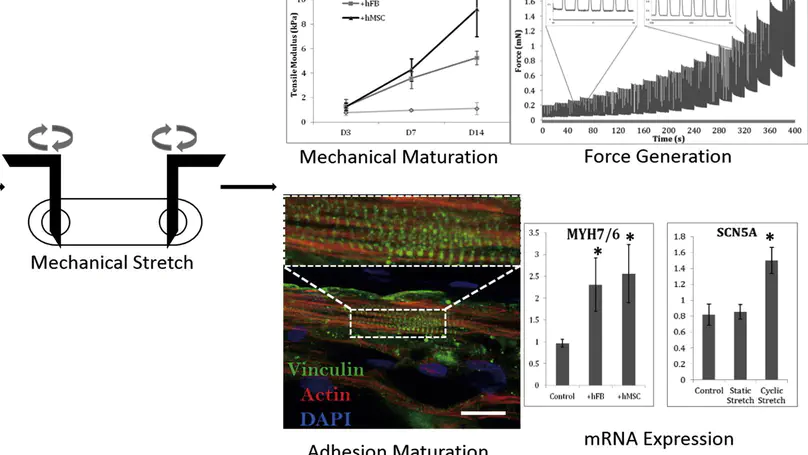
Cardiomyocytes derived from human embryonic stem cells (hESC-CMs) are regarded as a promising source for regenerative medicine, drug testing and disease modeling. Nevertheless, cardiomyocytes are immature in terms of their contractile structure, metabolism and electrophysiological properties. Here, we fabricate cardiac muscle strips by encapsulating hESC-CMs in collagen-based biomaterials. Supplementation of niche cells at 3% to the number of hESC-CMs enhance the maturation of the hESC-CMs in 3D tissue matrix. The benefits of adding mesenchymal stem cells (MSCs) are comparable to that of adding fibroblasts. These two cell types demonstrate similar effects in promoting the compaction and cell spreading, as well as expression of maturation markers at both gene and protein levels. Mechanical loading, particularly cyclic stretch, produces engineered cardiac tissues with higher maturity in terms of twitch force, elastic modulus, sarcomere length and molecular signature, when comparing to static stretch or non-stretched controls. The current study demonstrates that the application of niche cells and mechanical stretch both stimulate the maturation of hESC-CMs in 3D architecture. Our results therefore suggest that this 3D model can be used for in vitro cardiac maturation study.
Human osteoarthritic chondrocytes (hOACs) are characterized by their “dedifferentiated” and catabolic phenotype and lack the ability for restoring their inherent functions by themselves. Here we investigated whether extrinsically supplemented mechanical signal via compression loading would affect the phenotype of hOACs. Specifically, we applied cyclic compression loading on cultured hOACs-collagen constructs and measured the expression of the major chondrogenic factors, cell-matrix interaction molecules and matrix degradation enzymes. Dynamic compression loading stimulates the expression and nuclear localization of sox9 in hOACs and reduces the catabolic events via downregulated expression of collagenases. These results contribute to better understanding towards mechanoregulation of hOACs.
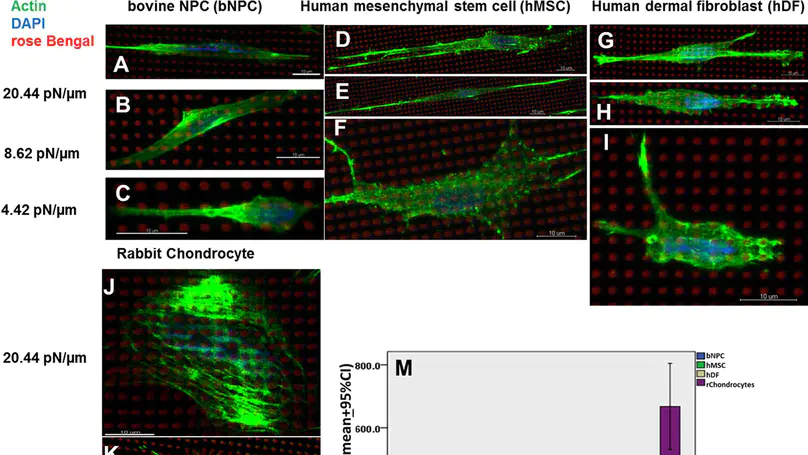
Engineering 3D microstructures with predetermined properties is critical for stem cell niche studies. We have developed a multiphoton femtosecond laser-based 3D printing platform, which generates complex protein microstructures in minutes. Here, we used the platform to test a series of fabrication and reagent parameters in precisely controlling the mechanical properties of protein micropillars. Atomic force microscopy was utilized to measure the reduced elastic modulus of the micropillars and transmission electron microscopy was used to visualize the porosity of the structures. The reduced elastic modulus of the micropillars associated positively and linearly with the scanning power. On the other hand, the porosity and pore size of the micropillars associated inversely and linearly with the scanning power and reagent concentrations. While keeping the elastic modulus constant, the stiffness of the micropillars was controlled by varying their height. Subsequently, the single cell traction forces of rabbit chondrocytes, human dermal fibroblasts, human mesenchymal stem cells and bovine nucleus pulposus cells (bNPCs) were successfully measured by culturing the cells on micropillar arrays of different stiffness. Our results showed that the traction forces of all groups showed positive relationship with stiffness and that the chondrocytes and bNPCs generated the highest and lowest traction forces, respectively.
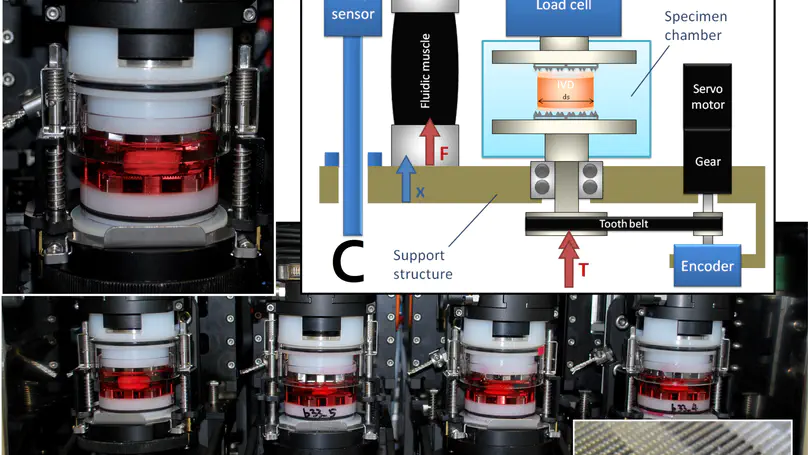
Mechanical loading has been shown to affect cell viability and matrix maintenance in the intervertebral disc (IVD) but there is no investigation on how cells survive mechanical stress and whether the IVD cells perceive mechanical loading as stress and respond by expression of heat shock proteins. This study investigates the stress response in the IVD in response to compressive loading. Bovine caudal disc organ culture was used to study the effect of physiological range static loading and dynamic loading. Cell activity, gene expression and immunofluorescence staining were used to analyze the cell response. Cell activity and cytoskeleton of the cells did not change significantly after loading. In gene expression analysis, significant up-regulation of heat shock protein-70 (HSP70) was observed in nucleus pulposus after two hours of loading. However, the expression of the matrix remodeling genes did not change significantly after loading. Similarly, expressions of stress response and matrix remodeling genes changed with application and removal of the dynamic loading. The results suggest that stress response was induced by physiological range loading without significantly changing cell activity and upregulating matrix remodeling. This study provides direct evidence on loading induced stress response in IVD cells and contributes to our understanding in the mechanoregulation of intervertebral disc cells. © 2016 Chooi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Matrix remodeling of cells is highly regulated by proteases and their inhibitors. Nevertheless, how would the chondrogenesis of mesenchymal stem cells (MSCs) be affected, when the balance of the matrix remodeling is disturbed by inhibiting matrix proteases, is incompletely known. Using a previously developed collagen microencapsulation platform, we investigated whether exposing chondrogenically differentiating MSCs to intracellular and extracellular protease inhibitors will affect the extracellular matrix remodeling and hence the outcomes of chondrogenesis. Results showed that inhibition of matrix proteases particularly the extracellular ones favors the phenotype of fibrocartilage rather than hyaline cartilage in chondrogenically differentiating hMSCs by upregulating type I collagen protein deposition and type II collagen gene expression without significantly altering the hypertrophic markers at gene level. This study suggests the potential of manipulating extracellular proteases to alter the outcomes of hMSC chondrogenesis, contributing to future development of differentiation protocols for fibrocartilage tissues for intervertebral disc and meniscus tissue engineering. © 2016 Han et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
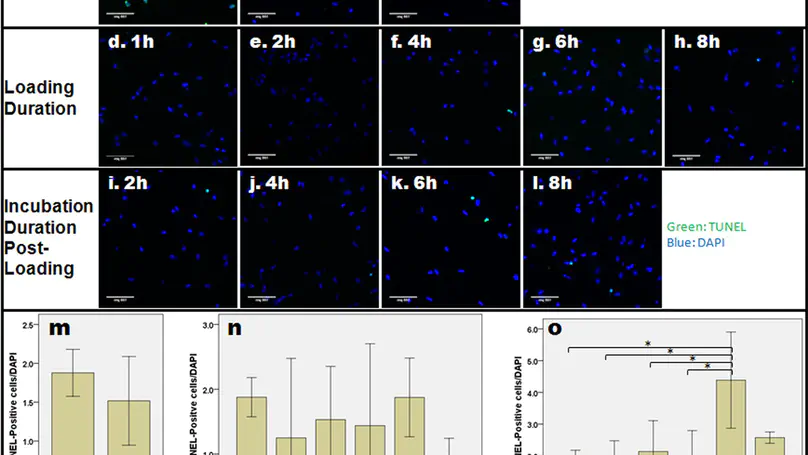
Cells protect themselves from stresses through a cellular stress response. In the interverebral disc, such response was also demonstrated to be induced by various environmental stresses. However, whether compression loading will cause cellular stress response in the nucleus pulposus cells (NPCs) is not well studied. By using an in vitro collagen microencapsulation model, we investigated the effect of compression loading on the stress response of NPCs. Cell viability tests, and gene and protein expression experiments were conducted, with primers for the heat shock response (HSR: HSP70, HSF1, HSP27 and HSP90), and unfolded protein response (UPR: GRP78, GRP94, ATF4 and CHOP) genes and an antibody to HSP72. Different gene expression patterns occurred due to loading type throughout experiments. Increasing the loading strain for a short duration did not increase the stress response genes significantly, but over longer durations, HSP70 and HSP27 were upregulated. Longer loading durations also resulted in a continuous upregulation of HSR genes and downregulation of UPR genes, even after load removal. The rate of apoptosis did not increase significantly after loading, suggesting that stress response genes might play a role in cell survival following mechanical stress. These results demonstrate how mechanical stress might induce and control the expression of HSR and UPR genes in NPCs.
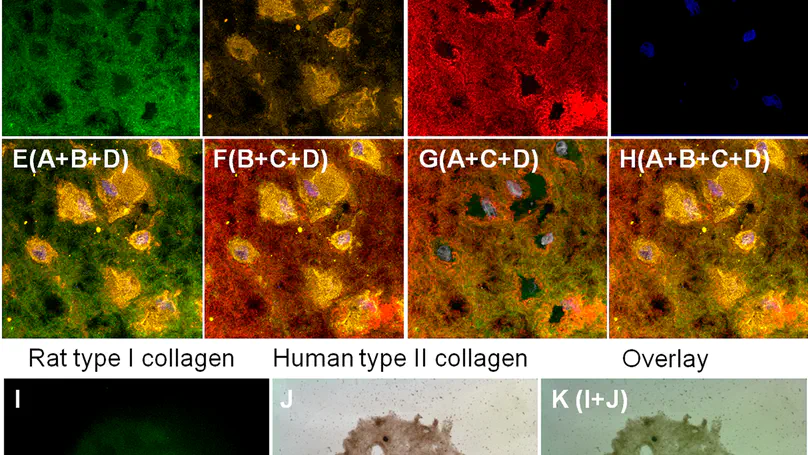
The stem cell niche, or microenvironment, consists of soluble, matrix, cell and mechanical factors that together determine the cellular fates and/or differentiation patterns of stem cells. Collagen and glycosaminoglycans (GAGs) are important scaffolding materials that can mimic the natural matrix niche. Here, we hypothesize that imposing changes in the scaffold composition or, more specifically, incorporating GAGs into the collagen meshwork, will affect the morphology, cytoskeletal organization and integrin expression profiles, and hence the fate of human mesenchymal stem cells (MSCs) upon the induction of differentiation. Using chondrogenesis as an example, we microencapsulated MSCs in three scaffold systems that had varying matrix compositions: collagen alone (C), aminated collagen (AC) and aminated collagen with GAGs (ACG). We then induced the MSCs to differentiate toward a chondrogenic lineage, after which, we characterized the cell viability and morphology, as well as the level of cytoskeletal organization and the integrin expression profile. We also studied the fate of the MSCs by evaluating the major chondrogenic markers at both the gene and protein level. In C, MSC chondrogenesis was successfully induced and MSCs that spread in the scaffolds had a clear actin cytoskeleton; they expressed integrin $α$2$β$1, $α$5 and $α$v; promoted sox9 nuclear localization transcription activation; and upregulated the expression of chondrogenic matrix markers. In AC, MSC chondrogenesis was completely inhibited but the scaffold still supported cell survival. The MSCs did not spread and they had no actin cytoskeleton; did not express integrin $α$2 or $α$v; they failed to differentiate into chondrogenic lineage cells even on chemical induction; and there was little colocalization or functional interaction between integrin $α$5 and fibronectin. In ACG, although the MSCs did not express integrin $α$2, they did express integrin $α$v and there was strong co-localization and hence functional binding between $α$v and fibronectin. In addition, vimentin was the dominant cytoskeletal protein in these cells, and the chondrogenic marker genes were expressed but at a much lower level than in the MSCs encapsulated in C alone. This work suggests the importance of controlling the matrix composition as a strategy to manipulate cell-matrix interactions (through changes in the integrin expression profile and cytoskeleton organization), and hence stem cell fates. © 2015 Elsevier Ltd.
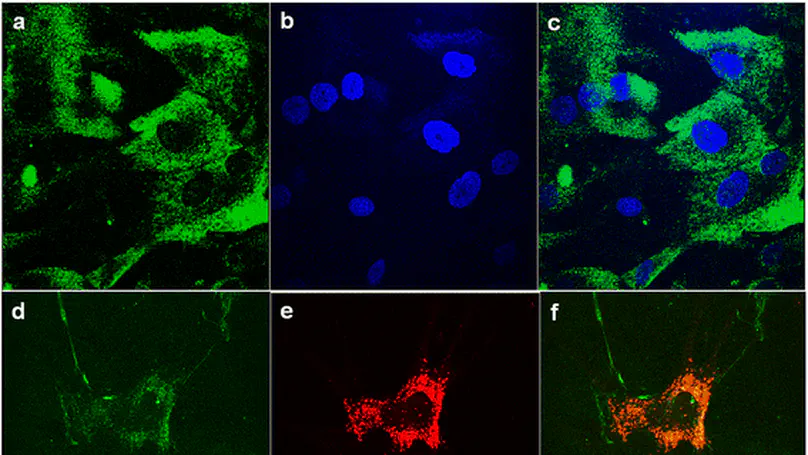
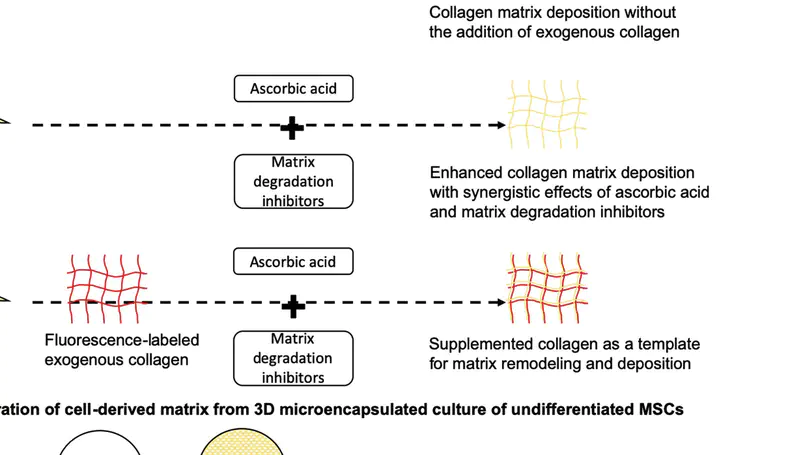
Introduction: Collagen is a widely used naturally occurring biomaterial for scaffolding, whereas mesenchymal stem cells (MSCs) represent a promising cell source in tissue engineering and regenerative medicine. It is generally known that cells are able to remodel their environment by simultaneous degradation of the scaffolds and deposition of newly synthesized extracellular matrix. Nevertheless, the interactions between MSCs and collagen biomaterials are poorly known, and the strategies enhancing the extracellular matrix deposition are yet to be defined. In this study, we aim to investigate the fate of collagen when it is in contact with MSCs and hypothesize that protease inhibition will enhance their extracellular deposition of collagen fibrils. Methods: Specifically, human MSCs (hMSCs) were exposed to fluorescence-labeled collagen with and without intracellular or extracellular protease inhibitors (or both) before tracing the collagen at both intracellular and extracellular spaces. Results: Collagen were internalized by hMSCs and degraded intracellularly in lysosomes. In the presence of protease inhibitors, both intracellular collagen fibril growth and extracellular deposition of collagen fibrils were enhanced. Moreover, protease inhibitors work synergistically with ascorbic acid, a well-known matrix deposition-enhancing reagent, in further enhancing collagen fibril deposition at the extracellular space. Conclusion: These findings provide a better understanding of the interactions between hMSCs and collagen biomaterials and suggest a method to manipulate matrix remodeling and deposition of hMSCs, contributing to better scaffolding for tissue engineering and regenerative medicine. © 2015 Han et al.
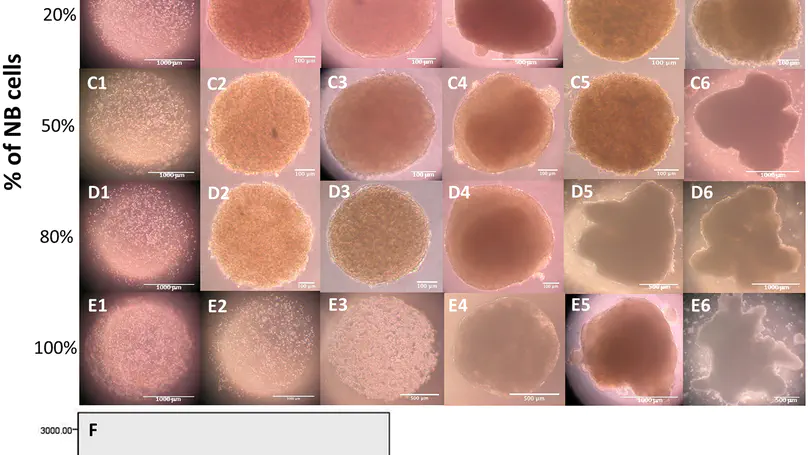
There is a growing trend for researchers to use in vitro 3D models in cancer studies, as they can better recapitulate the complex in vivo situation. And the fact that the progression and development of tumor are closely associated to its stromal microenvironment has been increasingly recognized. The establishment of such tumor supportive niche is vital in understanding tumor progress and metastasis. The mesenchymal origin of many cells residing in the cancer niche provides the rationale to include MSCs in mimicking the niche in neuroblastoma. Here we co-encapsulate and co-culture NBCs and MSCs in a 3D in vitro model and investigate the morphology, growth kinetics and matrix remodeling in the reconstituted stromal environment. Results showed that the incorporation of MSCs in the model lead to accelerated growth of cancer cells as well as recapitulation of at least partially the tumor microenvironment in vivo. The current study therefore demonstrates the feasibility for the collagen microsphere to act as a 3D in vitro cancer model for various topics in cancer studies. © 2015 Yeung et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in anymedium, provided the original author and source are credited.
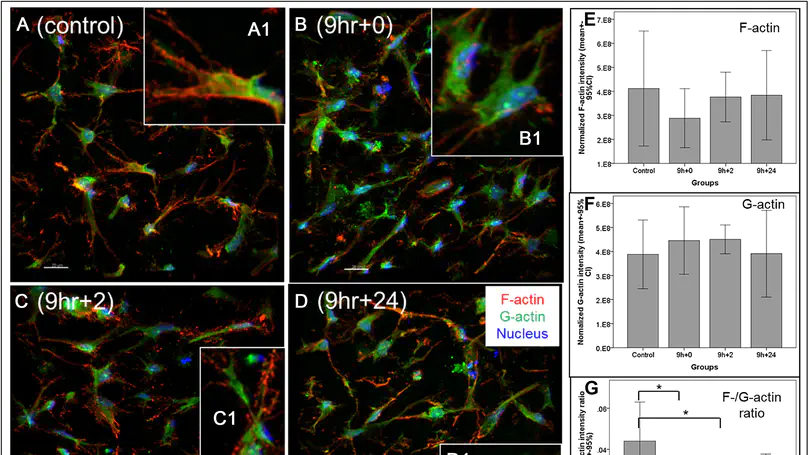
Cells are known to respond to multiple niche signals including extracellular matrix and mechanical loading. In others and our own studies, mechanical loading has been shown to induce the formation of cell alignment in 3D collagen matrix with random meshwork, challenging our traditional understanding on the necessity of having aligned substrates as the prerequisite of alignment formation. This motivates our adventure in deciphering the mechanism of loading-induced cell alignment and hence the discovery of the novel protrusive functional structure at the cell-matrix interface. Here we report the formation of mechanoresponsive, omni-directional and local matrix-degrading actin protrusions in human mesenchymal stem cells (hMSCs) microencapsulated in collagen following a shifted actin assembly/disassembly balance. These actin protrusive structures exhibit morphological and compositional similarity to filopodia and invadopodia but differ from them in stability, abundance, signaling and function. Without ruling out the possibility that these structures may comprise special subsets of filopodia and invadopodia, we propose to name them as mechanopodia so as to reveal their mechano-inductive mechanism. We also suggest that more intensive investigations are needed to delineate the functional significance and physiological relevance of these structures. This work identifies a brand new target for cell-matrix interaction and mechanoregulation studies. © 2015 Elsevier Ltd.
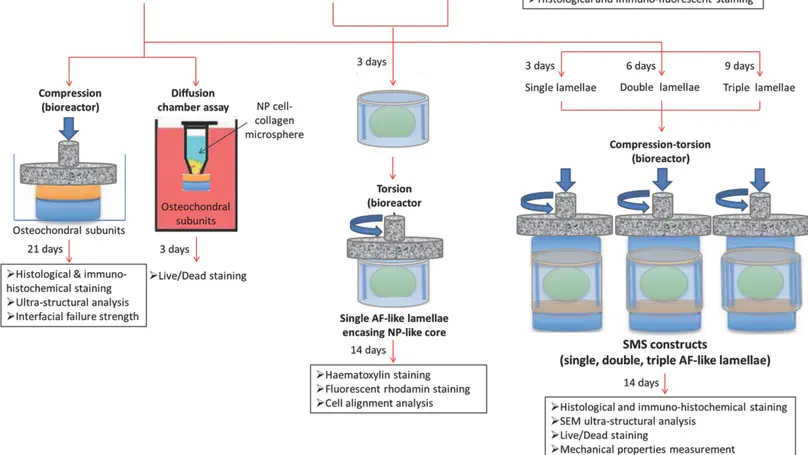
Intervertebral disc degeneration is an important clinical problem but existing treatments have significant drawbacks. The ability to bioengineer the entire spinal motion segment (SMS) offers hope for better motion preservation strategies but is extremely challenging. Here, fabrication of a multicomponent SMS construct with complex hierarchical organization from mesenchymal stem cells and collagen-based biomaterials, using a module-based integrative approach, is reported. The construct consists of two osteochondral subunits, a nucleus pulposus (NP-)-like core and a multi-lamellae annulus fibrosus (AF-)-like component. Chondrogenic medium is crucial for stabilizing the osteochondral subunits, which are shown to allow passive nutrient diffusion, while cyclic compression is necessary for better fiber matrix organization. Cells adhere, survive, and interact with the NP-like core. Cyclic torsional loading stimulates cell alignment in the AF-like lamellae and the number of lamellae affects the mechanical properties of the construct. This work represents an important milestone in SMS tissue engineering and provides a 3D model for studying tissue maturation and functional remodeling. The first bioengineered spinal motion segment construct simulating the native tissue with complex hierarchical organization is fabricated using a module-based integrative approach from mesenchymal stem cells and collagen-based biomaterials. This work represents an important milestone in bioengineering complex tissues and provides a 3D model for future screening of factors stimulating functional remodeling and tissue maturation. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
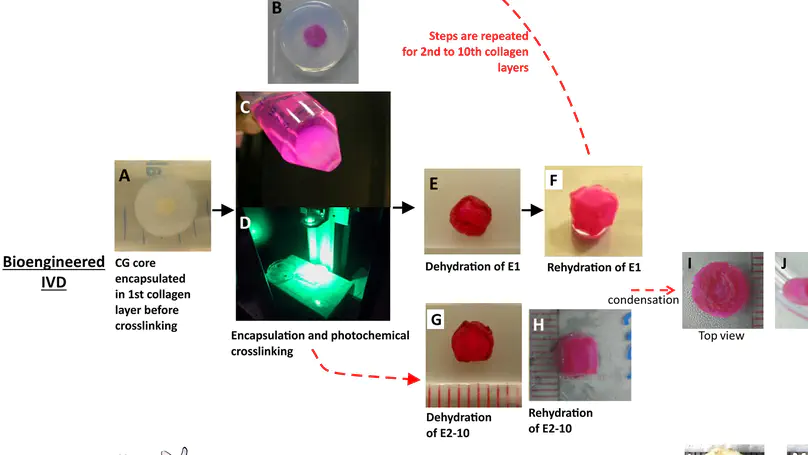
Tissue engineering offers high hopes for the treatment of intervertebral disc (IVD) degeneration. Whereas scaffolds of the disc nucleus and annulus have been extensively studied, a truly biomimetic and mechanically functional biphasic scaffold using naturally occurring extracellular matrix is yet to be developed. Here, a biphasic scaffold was fabricated with collagen and glycosaminoglycans (GAGs), two of the most abundant extracellular matrix components in the IVD. Following fabrication, the scaffold was characterized and benchmarked against native disc. The biphasic scaffold was composed of a collagen-GAG co-precipitate making up the nucleus pulposus-like core, and this was encapsulated in multiple lamellae of photochemically crosslinked collagen membranes comprising the annulus fibrosus-like lamellae. On mechanical testing, the height of our engineered disc recovered by $∼$82-89% in an annulus-independent manner, when compared with the 99% recovery exhibited by native disc. The annulus-independent nature of disc height recovery suggests that the fluid replacement function of the engineered nucleus pulposus core might mimic this hitherto unique feature of native disc. Biphasic scaffolds comprised of 10 annulus fibrosus-like lamellae had the best overall mechanical performance among the various designs owing to their similarity to native disc in most aspects, including elastic compliance during creep and recovery, and viscous compliance during recovery. However, the dynamic mechanical performance (including dynamic stiffness and damping factor) of all the biphasic scaffolds was similar to that of the native discs. This study contributes to the rationalized design and development of a biomimetic and mechanically viable biphasic scaffold for IVD tissue engineering. © 2015 Choy, Chan. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
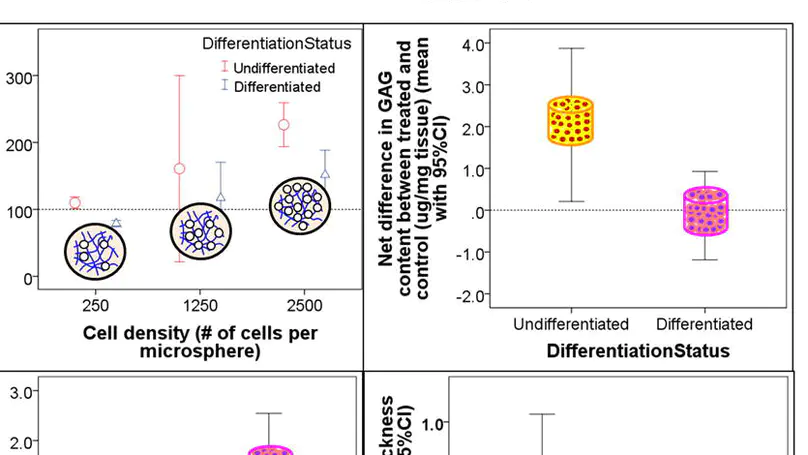
Mesenchymal stem cells (MSC) hold promise for cartilage repair. A microencapsulation technique was previously established to entrap MSC in collagen microspheres, and the collagen fibrous meshwork was found to be an excellent scaffold for supporting MSC survival, growth and differentiation. This study investigates the importance of cell density and differentiation status of MSC-collagen microspheres in cartilage repair. MSC were isolated from rabbit bone marrow and encapsulated in collagen microspheres. The effects of pre-differentiating the encapsulated MSC into chondrogenic lineages and different cell densities on cartilage repair were investigated in rabbits. Implantation of undifferentiated collagen-MSC microspheres formed hyaline-like cartilage rich in type II collagen and glycosaminoglycans (GAG) at 1 month post-implantation. By 6 months, hyaline cartilage rich in type II collagen and GAG, but negative for type I collagen, and partial zonal organization were found in both undifferentiated and chondrogenically differentiated groups in the high cell density group. The undifferentiated group and high cell density group significantly improved the O’Driscoll histological score. Moreover, the undifferentiated group significantly increased the GAG content. The mechanically differentiated group showed stiffer but thinner cartilage, while the undifferentiated group showed thicker but softer cartilage compared with their respective contra-lateral controls. This work suggests that a higher local cell density favors cartilage regeneration, regardless of the differentiation status of MSC, while the differentiation status of MSC does significantly affect regeneration outcomes. © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
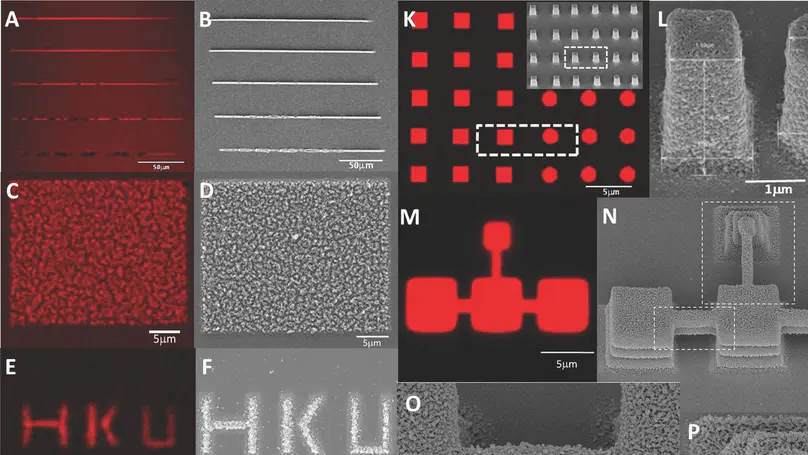
Femto-second laser-based free-writing of complex protein microstructures and micropatterns, with sub-micrometer features and controllability over voxel dimension, morphology, and porosity, is reported. Protein voxels including lines, spots, and micropillars are fabricated. Laser power, exposure time, z-position, protein and photosensitizer concentrations, but not scanning speed, are important controlling parameters. A lateral fabrication resolution of $≈$200 nm is demonstrated in 2D line voxels. 3D spot voxels are ellipsoids with 400 nm lateral and 1.5 $μ$m axial dimensions. An ascending z-stack scanning method to verify the theoretical axial optical resolution, delineate and enhance the axial fabrication resolution of 3D structures, including square prism and cylinder micropillars, is also reported. The micropillar array presents a simple “write-and-seed” and table platform for cell niche studies. Fibroblasts attach to, grow on, and express adhesion to molecules on micropillar arrays without the need of matrix coating. They exhibit a more “3D” morphology comparing with that in 2D monolayer cultures and physiological functions such as matrix deposition. This work presents an important milestone in engineering complex protein microstructures and micropatterns with sub-micrometer topological features to mimic the native matrix niche for cell-matrix interaction studies.
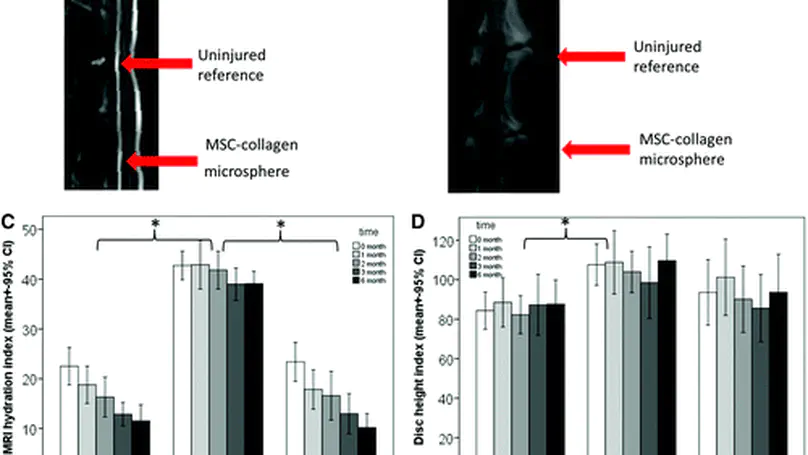
Mesenchymal stem cells (MSCs) have the potential to treat early intervertebral disc (IVD) degeneration. However, during intradiscal injection, the vast majority of cells leaked out even in the presence of hydrogel carrier. Recent evidence suggests that annulus puncture is associated with cell leakage and contributes to osteophyte formation, an undesirable side effect. This suggests the significance of developing appropriate carriers for intradiscal delivery of MSCs. We previously developed a collagen microencapsulation platform, which entraps MSCs in a solid microsphere consisting of collagen nanofiber meshwork. These solid yet porous microspheres support MSC attachment, survival, proliferation, migration, differentiation, and matrix remodeling. Here we hypothesize that intradiscal injection of MSCs in collagen microspheres will outperform that of MSCs in saline in terms of better functional outcomes and reduced side effects. Specifically, we induced disc degeneration in rabbits and then intradiscally injected autologous MSCs, either packaged within collagen microspheres or directly suspended in saline, into different disc levels. Functional outcomes including hydration index and disc height were monitored regularly until 6 months. Upon sacrifice, the involved discs were harvested for histological, biochemical, and biomechanical evaluations. MSCs in collagen microspheres showed advantage over MSCs in saline in better maintaining the dynamic mechanical behavior but similar performance in hydration and disc height maintenance and matrix composition. More importantly, upon examination of gross appearance, radiograph, and histology of IVD, delivering MSCs in collagen microspheres significantly reduced the risk of osteophyte formation as compared to that in saline. This work demonstrates the significance of using cell carriers during intradiscal injection of MSCs in treating disc degeneration. © 2014 Mary Ann Liebert, Inc.
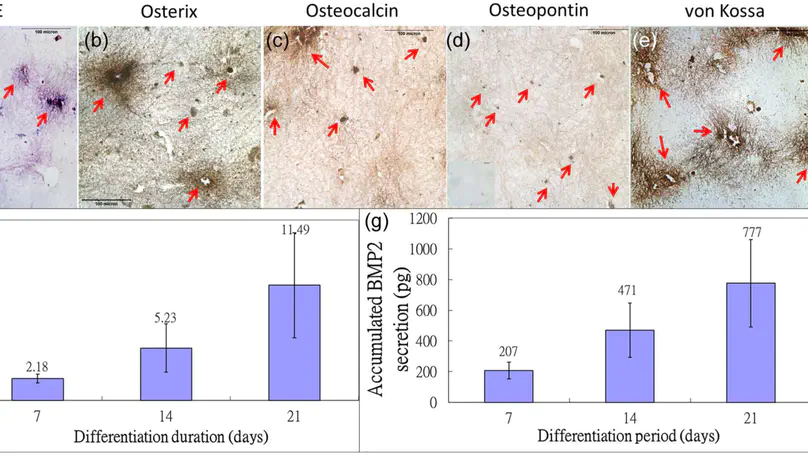
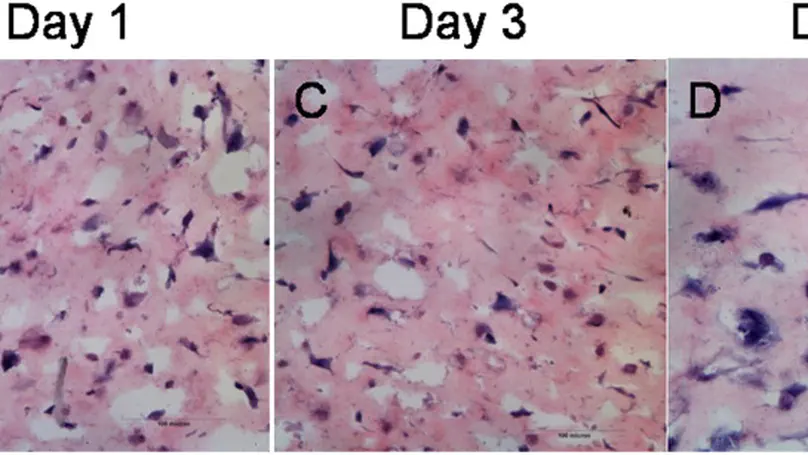
Mesenchymal stem/stromal cells (MSCs) and osteoblasts are important niche cells for hematopoietic stem cells (HSCs) in bone marrow osteoblastic niche. Here, we aim to partially reconstitute the bone marrow HSC niche in vitro using collagen microencapsulation for investigation of the interactions between HSCs and MSCs. Mouse MSCs (mMSCs) microencapsulated in collagen were osteogenically differentiated to derive a bone-like matrix consisting of osteocalcin, osteopontin, and calcium deposits and secreted bone morphogenic protein 2 (BMP2). Decellularized bone-like matrix was seeded with fluorescence-labeled human MSCs and HSCs. Comparing with pure collagen scaffold, significantly more HSCs and HSC-MSC pairs per unit area were found in the decellularized bone-like matrix. Moreover, incubation with excess neutralizing antibody of BMP2 resulted in a significantly higher number of HSC per unit area than that without in the decellularized matrix. This work suggests that the osteogenic differentiated MSC-collagen microsphere is a valuable three-dimensional in vitro model to elucidate cell-cell and cell-matrix interactions in HSC niche. © The Author(s) 2013.
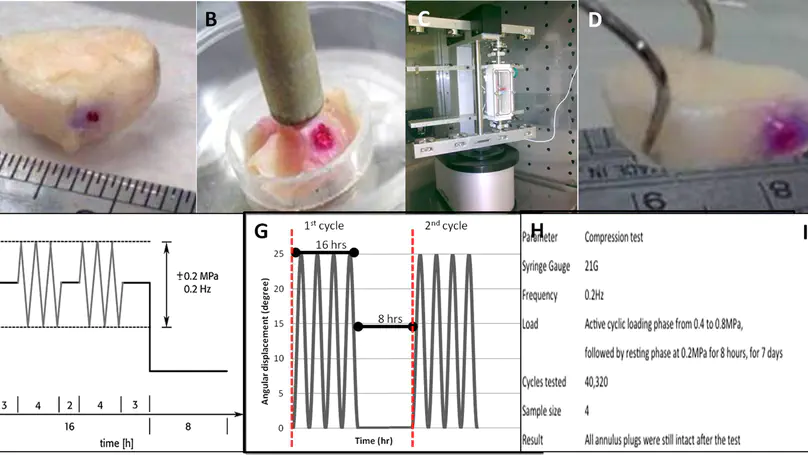
Intra-disc injection of mesenchymal stem cells (MSCs) to treat disc degeneration may lead to unfavorable complications, particularly osteophyte formation. Development of an effective method to block the injection portal, prevent the leakage of injected cells and materials and, hence, prevent osteophyte formation is of the utmost importance before MSC-based therapies can be applied in a clinical setting. Here we seek to alleviate the cell leakage problem and the associated complication osteophyte formation by developing an injectable annulus plug to block the injection portal during intra-disc delivery. Specifically, we fabricated a needle-shaped collagen plug by photochemical crosslinking and successfully delivered it intra-discally, in association with MSCs in collagen microsphere carriers, using a custom-made delivery device. The mechanical performance of the plug and its effectiveness in reducing cell leakage were evaluated ex vivo under compression and in torsion push-out tests. The results demonstrate that the plug survived physiologically relevant loadings and significantly reduced leakage and enhanced retention of the injected materials. Finally, a pilot in vivo study in rabbits was conducted to evaluate the performance of the plug. Microcomputed tomography imaging and histology revealed that the plug significantly reduced osteophyte formation. This work suggests the potential of the annulus plug as an adjunct or annulus closure device for intra-disc delivery of cells and materials. © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
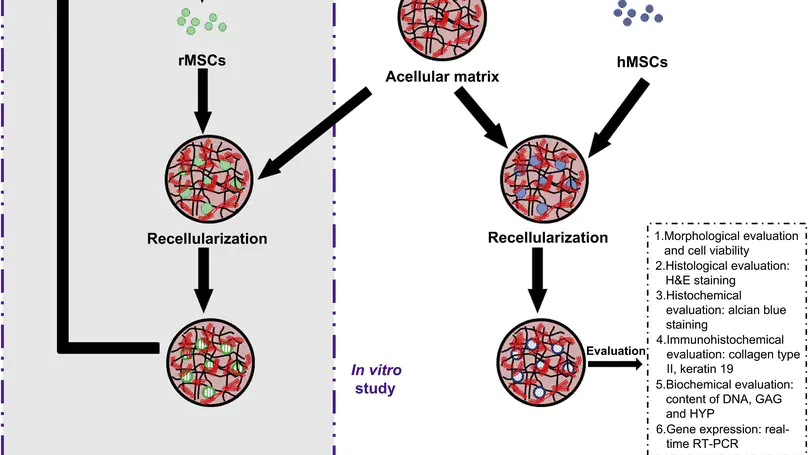
Recent attempts to treat disc degeneration with mesenchymal stem cells (MSCs) showed encouraging results. Differentiating MSCs towards nucleus pulposus cell (NPC)-like lineages represents a speculative mechanism. Niche factors including hypoxia, growth factors and cell-cell interactions have been suggested but the matrix niche factor has not been studied. Our collagen microencapsulation provides a 3D model to study matrix niche as it enables the encapsulated cells to remodel the template matrix. We previously demonstrated the chondro-inductive role of of chondrocytes-derived matrix in MSCs and showed that NPCs maintained their phenotype and remodeled the template matrix of collagen microspheres into a glycosaminoglycan (GAG)-rich one. Here we aim to study the effects of NPC-derived matrix on MSC differentiation towards NPC-like lineages by firstly producing an NPC-derived matrix in collagen microspheres, secondly optimizing a decellularization protocol to discard NPCs yet retaining the matrix, thirdly repopulating the acellular NPC-derived matrix with MSCs and fourthly evaluating their phenotype. Finally, we injected these microspheres in a pilot rabbit disc degeneration model. Results showed that NPCs survived, maintained their phenotypic markers and produced GAGs. A decellularization protocol with maximal removal of the NPCs, minimal loss in major matrix components and partial retention of NPC-specific markers was identified. The resulting acellular matrix supported MSC survival and matrix production, and up-regulated the gene expression of NPC markers including type II collagen and glypican 3. Finally, injection of MSC in these microspheres in rabbit degenerative disc better maintained hydration level with more pronounced staining of GAGs and type II collagen than controls. © 2013 Elsevier Ltd.
Controlling cell organization is important in tissue engineering. Guidance by aligned features on scaffolds or stimulation by physical signals can be used to induce cell alignment. We have previously demonstrated a preferred alignment of human MSCs (hMSCs) along the compression loading axis in 3D collagen construct. In this study, we aim to investigate the collagen concentration dependence of the compression-induced hMSC organization. Results demonstrated that the compression-induced alignment and elongation of hMSCs exhibited a biphasic dose-dependent relationship with collagen concentration, and associated well with both collagen ligand density and elastic modulus of the constructs. Moreover, collagen concentration and compression loading significantly affected the expression level of integrin beta 1 and antibody neutralization against this molecule aborted the compression-induced alignment and elongation responses. © 2012 Wiley Periodicals, Inc. J Biomed Mater Res Part A, 2013. Copyright © 2012 Wiley Periodicals, Inc.
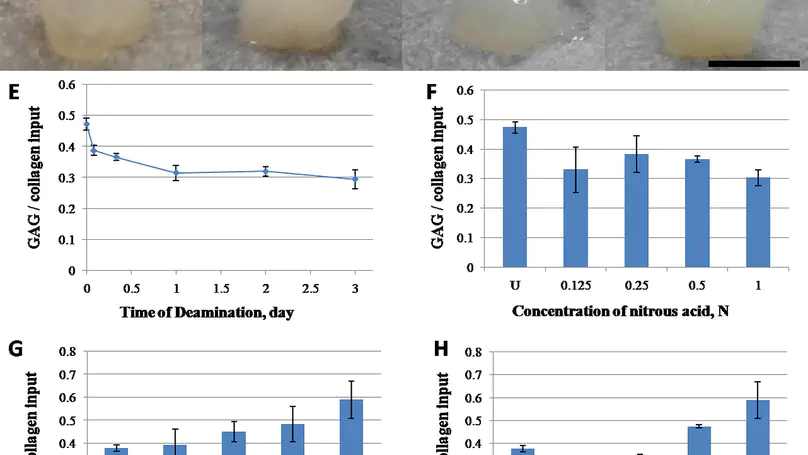
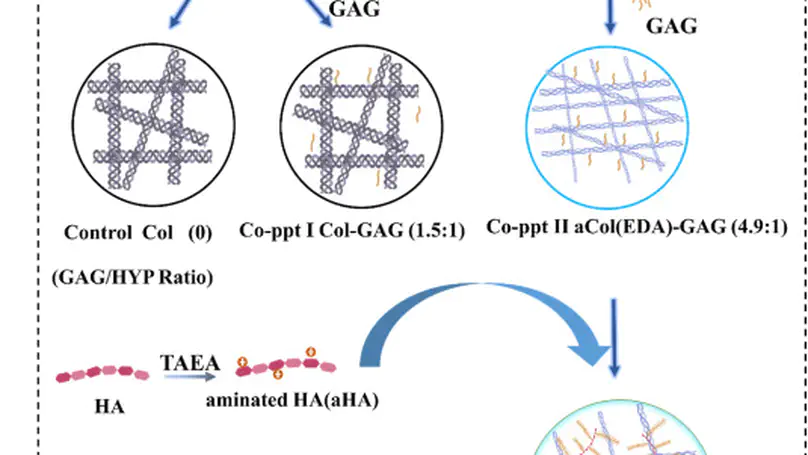
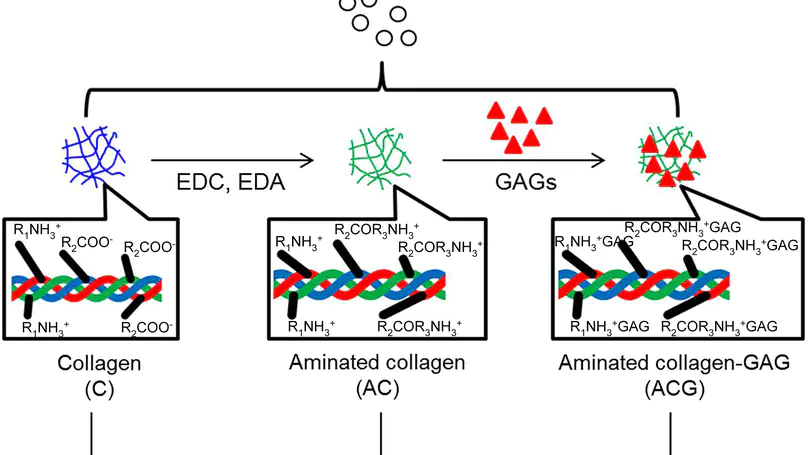
Being prevalent extracellular matrix components, collagen and glycosaminoglycan (GAG) are co-precipitated as scaffolds for tissue regeneration. However, the amount of GAG incorporated and its long-term retention present a persistent problem. In this study, chemical modifications, namely deamination, methylation and amination, were used to alter the net charge of collagen prior to fabrication of collagen-GAG co-precipitate. While most GAGs were lost in the untreated group and the deaminated group within 1 day, methylation and amination of collagen retained over 20% and 40% GAG after 6 days, respectively. Moreover, over 60% of GAG retention was achieved in the aminated group after cell seeding for 8 days. Furthermore, amination of collagen increased the GAG/hydroxyproline ratio in the co-precipitate to $>$4.5, approaching that of native nucleus pulposus. Ultrastructural analysis showed that the aminated group contains abundant granular substances resembling the extracellular matrix of native nucleus pulposus. Despite lower initial cell adhesion than untreated, all modified scaffolds promoted proliferation of human mesenchymal stem cells (hMSCs) and showed $>$95% cell viability at all time points. Cell morphology was distinct among the different groups, being round in the untreated control and methylated groups but elongated in deaminated and aminated groups. hMSCs adhered to scaffolds via collagen receptor integrin $α$2$β$1 in all groups, while all but the aminated group showed extensive expression of the general matrix receptor integrin $α$v. This work reports an effective method, namely amination of collagen, to improve GAG incorporation and retention in collagen-GAG co-precipitates, facilitating the fabrication of GAG-rich collagenous scaffold for intervertebral disc tissue engineering. © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
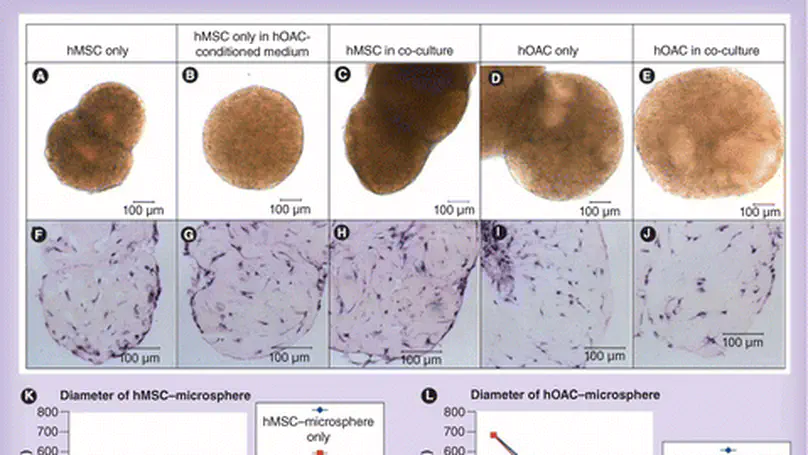
Aim: Mesenchymal stem cell (MSC)-based therapy presents a promising approach for treating osteoarthritis (OA). However, the molecular interactions between MSCs and OA chondrocytes (OACs) are not known. This study aims to investigate the bidirectional interactions between human MSCs (hMSCs) and human OACs (hOACs) in a 3D co-culture system. Materials & methods: hMSC-collagen microspheres were cultured in hOAC-conditioned medium or co-cultured with hOAC-collagen microspheres. Growth characteristics, glycosaminoglycan (GAG) production, gene expression of major OA-associated chondrogenic markers, including SOX9, COL2A1, ACAN and MMP13, were investigated in both cell types. Results: Both the conditioned medium and the co-culture induced MSC chondrogenesis with enhanced GAG production, SOX9 gene and protein expression, and gene expression of ACAN and COL2A1. Meanwhile, the co-culture also induced hOACs to partially resume the lost chondrogenic phenotype as shown by reduced proliferation, enhanced GAG production when hMSCs were chondrogenically predifferentiated, and reduced MMP13 gene expression. Conclusion: This work suggests that 3D co-culture of hMSCs and hOACs is mutually beneficial to each other, suggesting the potential therapeutic effect of delivering hMSC in scaffolds directly to OA defects. © 2013 Future Medicine Ltd.
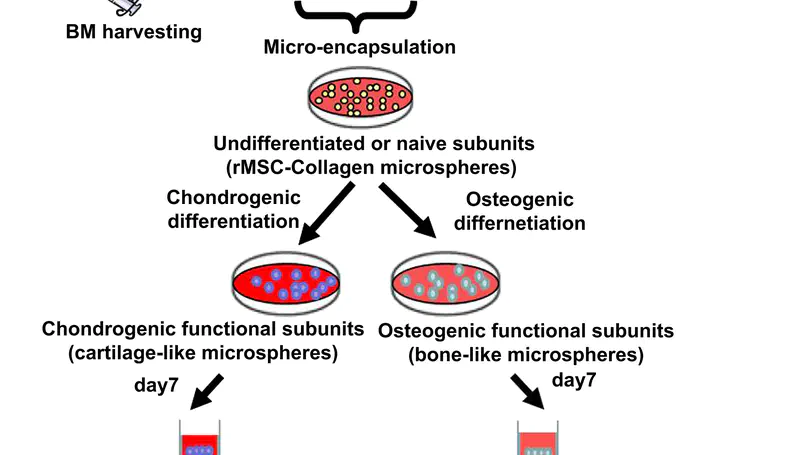
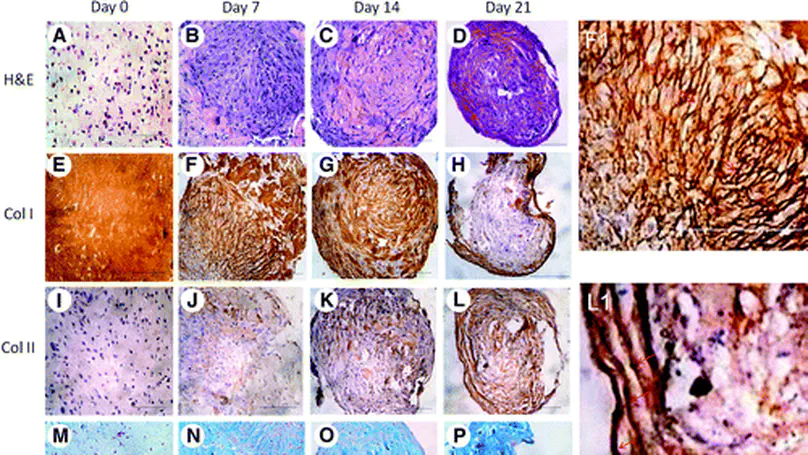
Creating biological interfaces between mechanically dissimilar tissues is a key challenge in complex tissue engineering. An osteochondral interface is essential in preventing mechanical failure and maintaining normal function of cartilage. Despite tremendous efforts in developing osteochondral plugs, formation of the osteochondral interface with proper zonal organization has not yet been reported. Here, we present a mesenchymal stem cell-collagen microsphere-based approach for complex tissue engineering and demonstrate in vitro formation of a stem cell-derived osteochondral interface with calcified cartilage interface separating a non-calcified cartilage layer and an underlying bone layer. Cells at the interface region are hypertrophic chondrocytes while the extracellular matrix in this region contains collagen type II and X, calcium deposits and vertically running fibers. The simultaneous presence of appropriate medium and configuration during co-culture is necessary for the interface formation. © 2010 Elsevier Ltd.
Mesenchymal stem cell (MSC)-based engineering is promising for cartilage repair. However, the compositional mechanical relationship of the engineered structures has not been extensively studied, given the importance of such relationship in native cartilage tissues. In this study, a novel human MSC-collagen microsphere system was used to study the compositional mechanical relationship during in vitro chondrogenic differentiation using histological and biochemical methods and a microplate compression assay. The mechanical property was found positively correlating with newly deposited cartilage-relevant matrices, glycosaminoglycan, and type II collagen, and with the collagen crosslinker density, in agreement with the presence of thick collagen bundles upon structural characterization. On the other hand, the mechanical property negatively correlates with type I collagen and total collagen, suggesting that the initial collagen matrix scaffold of the microsphere system was being remodeled by the differentiating human MSCs. This study also demonstrated the application of a simple, sensitive, and nondestructive tool for monitoring the progression of chondrogenic differentiation of MSCs in tissue-engineered constructs and therefore contributes to future development of novel cartilage repair strategies. © Mary Ann Liebert, Inc. 2011.
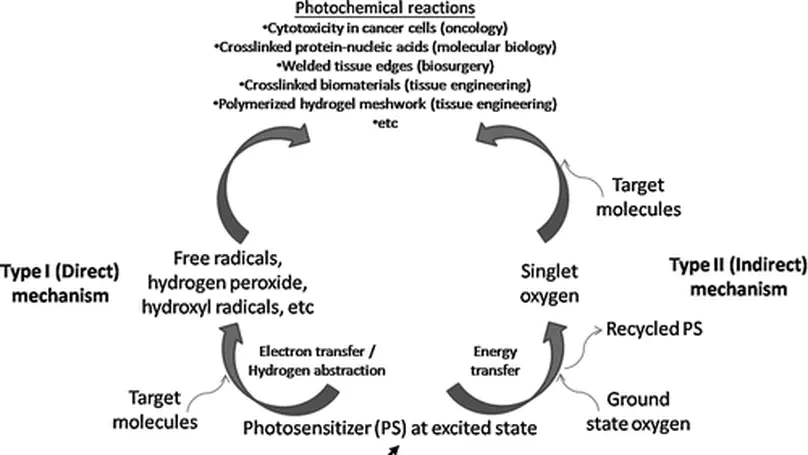
Photochemistry is the study of photochemical reactions between light and molecules. Recently, there have been increasing interests in using photochemical reactions in the fields of biomaterials and tissue engineering. This work revisits the components and mechanisms of photochemistry and reviews biomedical applications of photochemistry in various disciplines, including oncology, molecular biology, and biosurgery, with particular emphasis on tissue engineering. Finally, potential toxicities and research opportunities in this field are discussed.
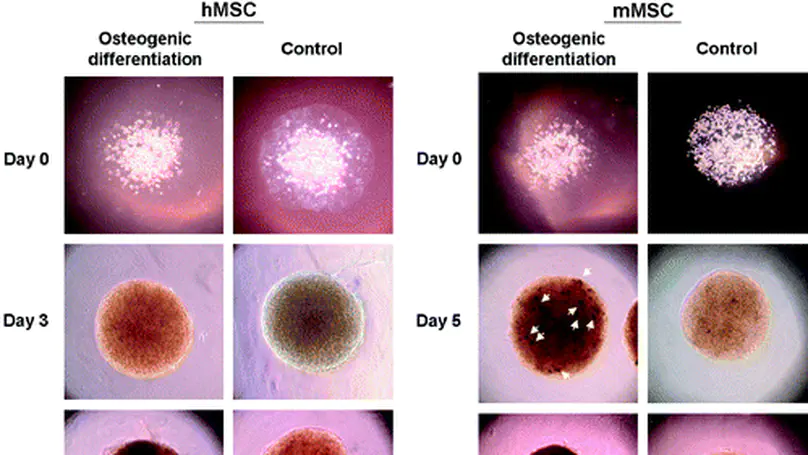
There is a demonstrated clinical need for alternatives of autologous fresh bone graft with excellent biological performance in osteoconductivity, osteoinductivity, and osteogenicity. We previously developed a collagen microencapsulation technology entrapping bone marrow-derived mesenchymal stem cells (MSCs) in a biomimetic collagen fiber meshwork and produced injectable collagen-MSC microspheres. In this study, we hypothesize that injectable microspheres with osteoconductivity, osteogenicity, and osteoinductivity can be fabricated by differentiating the encapsulated MSCs, from either human or mouse sources, toward osteogenic lineages in these three-dimensional microspheres. The osteogenicity, osteoconductivity, and osteoinductivity of the microspheres were evaluated in vitro. Osteogenic markers of the differentiating MSCs including alkaline phosphatase and calcium deposition showed positive staining. Osteoconductivity of the collagen meshwork in the microsphere was demonstrated by the presence of calcium phosphate deposits among the collagen fibers and by the significantly increased calcium content extracted from the microspheres. Moreover, osteoinductivity of the MSC-encapsulated microspheres was demonstrated by the ability to induce osteogenic differentiation of undifferentiated MSCs in both contact and noncontact coculture. This study contributes toward the future development of injectable alternatives for fresh bone grafts using autologous MSCs. © 2010, Mary Ann Liebert, Inc.
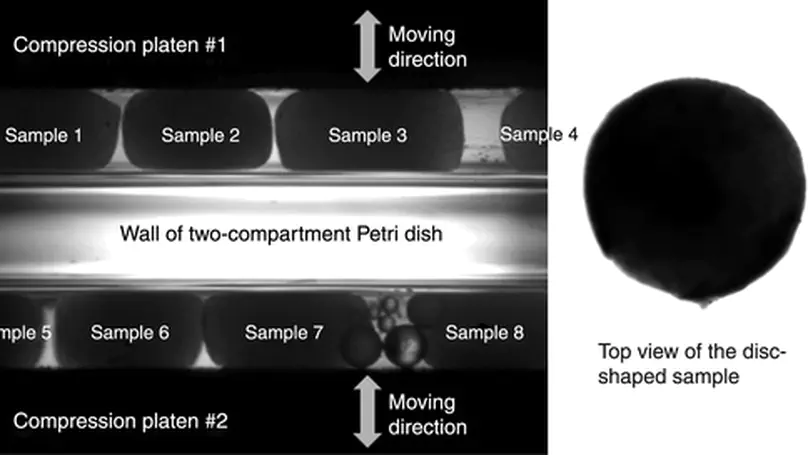
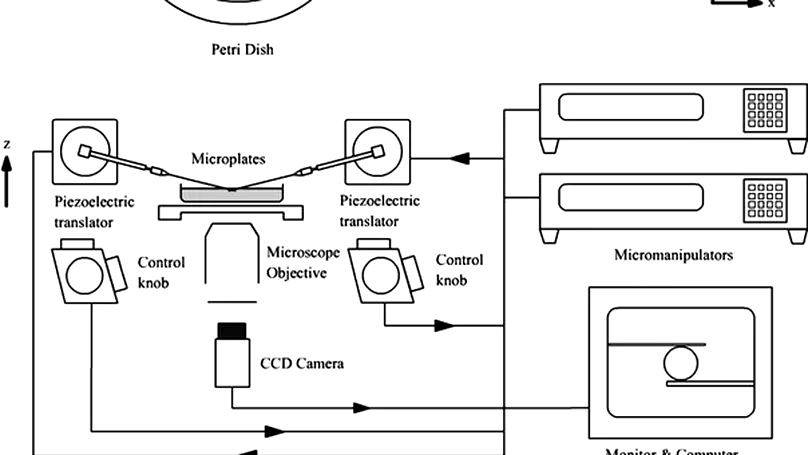
Mechanical signal is important for regulating cellular activities, including proliferation, metabolism, matrix production, and orientation. Bioreactors with loading functions can be used to precondition cells in three-dimensional (3D) constructs so as to study the cellular responses to mechanical stimulation. However, full-scale bioreactor is not always an affordable option considering the high cost of equipments and the liter-sized medium with serum and growth factor supplements. In this study, a custom-built loading system was developed by coupling a conventional camera-equipped inverted research microscope with two micromanipulators. The system was programmed to deliver either cyclic compressive loading with different frequencies or static compressive loading for 1 week to investigate the cellular responses of human mesenchymal stem cells (hMSCs) entrapped in a 3D construct consists of reconstituted collagen fibers. Cellular properties, including their alignment, cytoskeleton, and cell metabolism, and properties of matrix molecules, such as collagen fiber alignment and glycosaminoglycan deposition, were evaluated. Using a MatLab-based image analysis program, reorientation of the entrapped cells from a random distribution to a preferred alignment along the loading direction in constructs with both static and cyclic compression has been demonstrated, but no such alignment was found in the free-floating controls. Fluorescent staining on filamentous actin cytoskeleton also confirmed the finding. Nevertheless, the collagen fiber meshwork entrapping the hMSCs remained randomly distributed, and no change in cellular metabolism and glycosaminoglycans production was noted. The current study provides a simple and affordable option toward setting up a mechanoregulation facility based on existing laboratory equipments and sheds new insights on the effect of mechanical loading on the alignment of hMSCs in 3D collagen constructs. Copyright © 2010, Mary Ann Liebert, Inc.
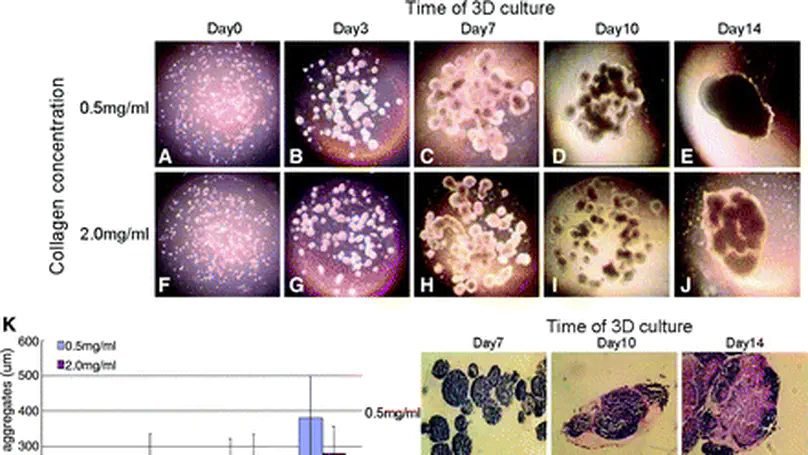
Embryonic stem (ES) cells are pluripotent cells with great potential in regenerative medicine. However, controlling their differentiation toward homogeneous lineages is challenging. In this study, we aim to investigate the effects of reconstituted 3D collagen matrix on the fates of mouse ES (mES) cells before and after induction for chondrogenic differentiation. Specifically, mES cells were encapsulated and cultured in 3D collagen microspheres and exposed to induction signals at different time points. Growth characteristics and differentiation status of mES cells were then evaluated. Collagen microspheres provided a suitable microenvironment supporting mES cell growth and maintaining their undifferentiated status for certain period of time. At later time points, the proportion of undifferentiated mES cells gradually decreased, accompanied by increasing proportions of mesenchymal progenitor cells. This suggests the inductive role of collagen matrix in differentiating mES cells toward mesenchymal lineages. Moreover, a lower initial collagen monomer concentration facilitated the differentiation of mES cells into chondrogenic lineages, while induction at a later time point associated with a more advanced stage of chondrogenic differentiation. This indicates that both the initial collagen concentration and the time to induce differentiation significantly affected the fates of mES cells. This study contributes to future development of ES cell-based therapies. © Copyright 2009, Mary Ann Liebert, Inc.

Glial cell line-derived neurotrophic factor (GDNF) is a potent neurotrophic factor. Development of drug delivery technologies facilitating controlled release of GDNF is critical to applying GDNF in treating neurodegenerative diseases. We previously developed 3D collagen microspheres and demonstrated enhanced GDNF secretion after encapsulation of HEK293 cells, which were transduced to overexpress GDNF in these microspheres. However, the entrapped HEK293 cells were able to migrate out of the collagen microspheres, making it undesirable for clinical applications. In this report, we investigate two new carrier designs, namely collagen-alginate composite gel and collagen microspheres embedded in alginate gel in preventing cell leakage, maintaining cell growth and controlling GDNF secretion in the HEK293 cells. We demonstrated that inclusion of alginate gel in both designs is efficient in preventing cell leakage to the surrounding yet permitting the GDNF secretion, although the cellular growth rate is reduced in an alginate concentration dependent manner. Differential patterns of GDNF secretion in the two designs were demonstrated. The collagen-alginate composite gel maintains a more or less constant GDNF secretion over time while the collagen microspheres embedded in alginate gel continue to increase the secretion level of GDNF over time. This study contributes towards the development of cell-based GDNF delivery devices for the future therapeutics of neurodegenerative diseases. © 2008 Elsevier Ltd. All rights reserved.
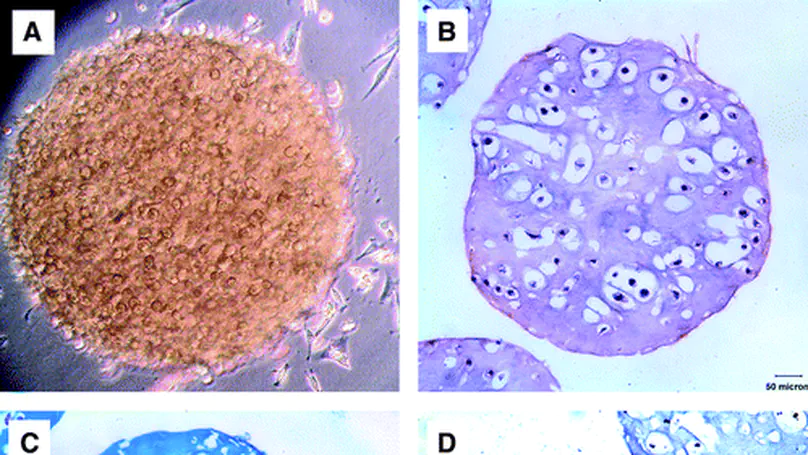
Extracellular matrix (ECM) partially constitutes the stem cell niche. Reconstituting the ECM niche in a three-dimensional (3D) configuration will significantly enhance our understanding of how stem cells interact with and respond to the ECM niche. In this study, we aimed to reconstitute a glycosaminoglycan (GAG)-rich ECM using a microencapsulation technology, produce acellular matrix using a decellularization technique, and investigate the effect of acellular matrix on stem cell fate by repopulating the matrix with human mesenchymal stem cells (hMSCs). We demonstrated that porcine chondrocytes were able to deposit a GAG-rich ECM within the 3D collagen microsphere. All decellularization treatment groups resulted in significant removal of chondrocyte nuclei, but acellular matrix was only achieved using 2% sodium deoxycholate. Nevertheless, decellularization resulted in significant loss in GAG content in almost all treatment groups, and the 2% sodium deoxycholate group was able to preserve about 40% of the GAGs compared with the control group. We further demonstrated that hMSCs seeded onto the decellularized microspheres were able to survive and penetrate into the centre, while hMSCs seeded in the acellular matrix showed positive immunostaining against sox9, indicating that they may be differentiating toward the chondrogenic lineage without the need to supplement the chondrogenic differentiation medium. © 2009 Mary Ann Liebert, Inc.
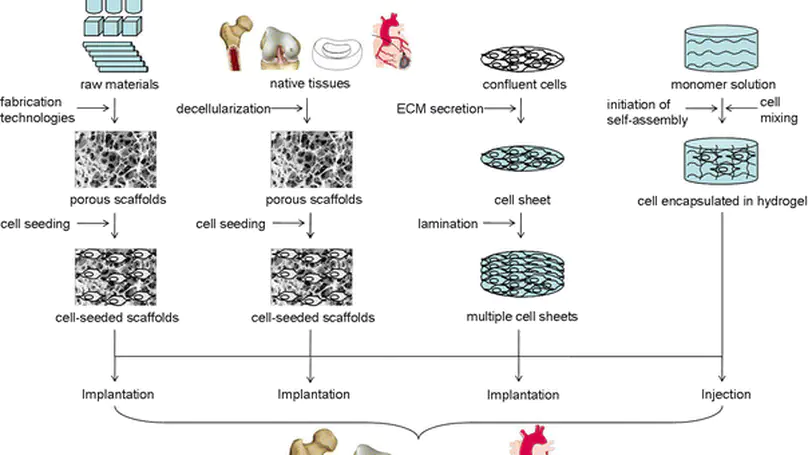
Scaffolds represent important components for tissue engineering. However, researchers often encounter an enormous variety of choices when selecting scaffolds for tissue engineering. This paper aims to review the functions of scaffolds and the major scaffolding approaches as important guidelines for selecting scaffolds and discuss the tissue-specific considerations for scaffolding, using intervertebral disc as an example. © 2008 Springer-Verlag.
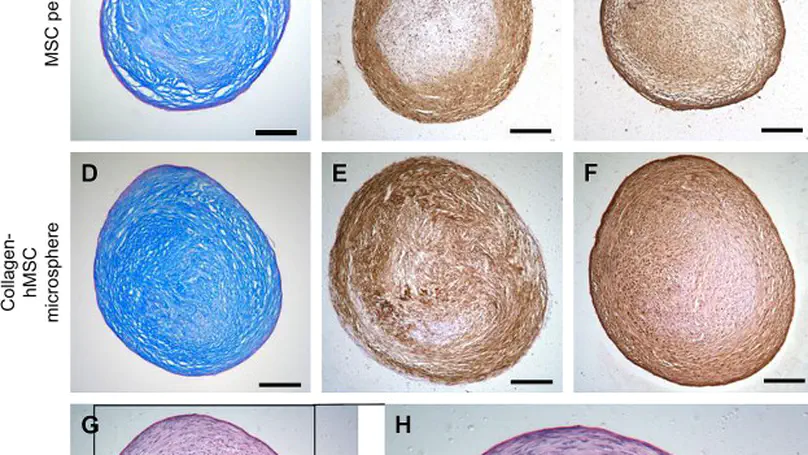
Given the inadequacies of existing repair strategies for cartilage injuries, tissue engineering approach using biomaterials and stem cells offers new hope for better treatments. Recently, we have fabricated injectable collagen-human mesenchymal stem cell (hMSC) microspheres using microencapsulation. Apart from providing a protective matrix for cell delivery, the collagen microspheres may also act as a bio-mimetic matrix facilitating the functional remodeling of hMSCs. In this study, whether the encapsulated hMSCs can be pre-differentiated into chondrogenic phenotype prior to implantation has been investigated. The effects of cell seeding density and collagen concentration on the chondrogenic differentiation potential of hMSCs have been studied. An in vivo implantation study has also been conducted. Fabrication of cartilage-like tissue micro-masses was demonstrated by positive immunohistochemical staining for cartilage-specific extracellular matrix components including type II collagen and aggrecan. The meshwork of collagen fibers was remodeled into a highly ordered microstructure, characterized by thick and parallel bundles, upon differentiation. Higher cell seeding density and higher collagen concentration favored the chondrogenic differentiation of hMSCs, yielding increased matrix production and mechanical strength of the micro-masses. These micro-masses were also demonstrated to integrate well with the host tissue in NOD/SCID mice. © 2008 Elsevier Ltd. All rights reserved.
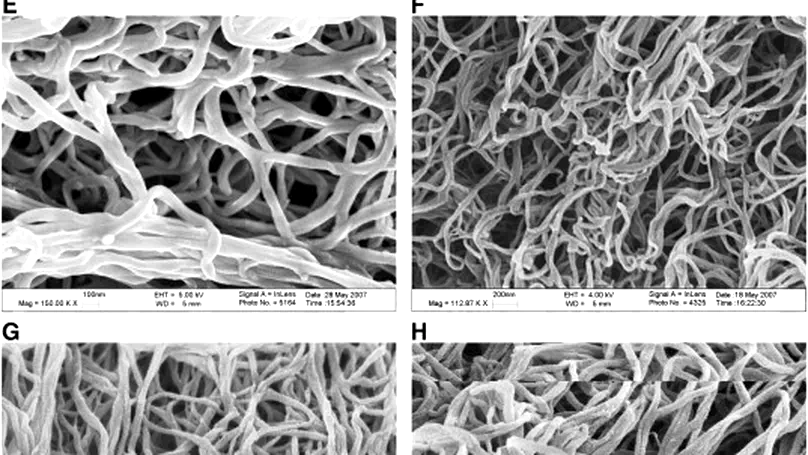
Protein compatibility is important for protein drug delivery using microsphere-based devices. Collagen has excellent protein compatibility but has poor mechanical stability for microsphere fabrication and open meshwork for controlled release. In this study, a protein-compatible fabrication method for injectable collagen microspheres has been developed. The surface morphology, interior microstructure and protein release characteristics of collagen microspheres were investigated. Moreover, effects of photochemical crosslinking on these characteristics were also studied. Finally, the mechanisms governing the protein release and the retention of protein bioactivity were studied. Stable and injectable collagen microspheres consisting of nano-fibrous meshwork were successfully fabricated under ambient conditions in an organic solvent and crosslinking reagent-free manner. These microspheres have open meshwork and showed large initial burst and rapid release of proteins. Photochemical crosslinking significantly reduced the initial burst effect and controlled the protein release in a photosensitizer dose-dependent manner without significantly altering the mesh size. We further demonstrated that there was significantly higher protein retention within the photochemically crosslinked collagen microspheres as compared with the uncrosslinked, suggesting a secondary retention mechanism. Lastly, both surfactant treatment and photochemical crosslinking did not compromise the bioactivity of the encapsulated proteins. In summary, this study reports a novel collagen microsphere-based protein delivery system and demonstrates the possibility to use photochemical crosslinking as the secondary retention mechanism for proteins. © 2008 Elsevier B.V. All rights reserved.
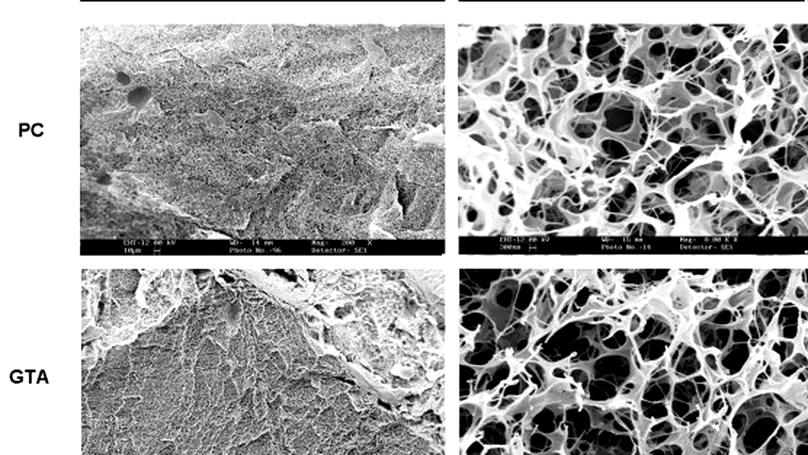
Photochemical crosslinking is an emerging technique able to modify the physicochemical properties of collagen. However, whether this technique can be used to modify collagen-based structures for drug delivery has not been studied. This study demonstrated that the microporous structure of photochemically crosslinked collagen was affected by rose Bengal and laser energy level. Using the optimized process parameters, the authors fabricated photochemically crosslinked collagen structures encapsulated with sample proteins and demonstrated that photochemical crosslinking reduced the initial burst effect and protein release without compromising the protein bioactivity. The fiber meshwork in collagen structures was also characterized, and it was found that photochemical crosslinking did not significantly alter the mesh size. This study reports the effects of photochemical crosslinking on the microstructure of collagen structures and suggests the feasibility of using photochemically crosslinked collagen structures for controlled protein release. © 2008 Acta Materialia Inc.
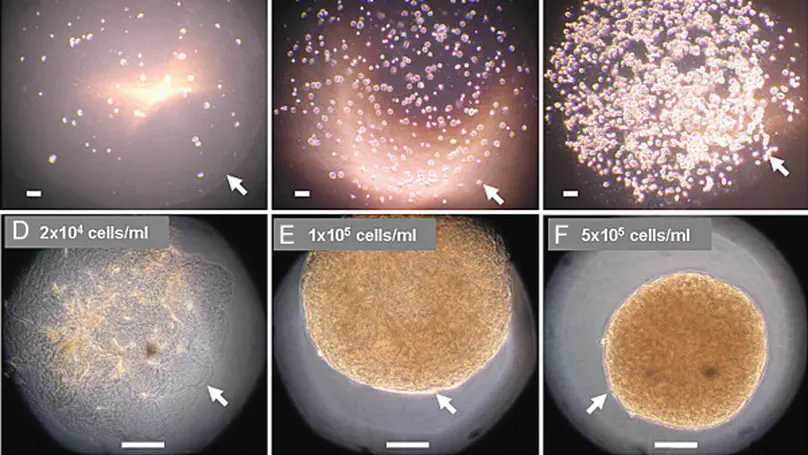
Hydrogel-based microspheres are commonly used for drug and cell delivery in regenerative medicine. Characterization of their physical and mechanical properties is important in monitoring their quality during fabrication and in predicting their performance upon injection. However, existing methods have limitations in measuring these micron-sized, soft and viscoelastic spherical structures. In this study, a protocol is developed to measure the elastic modulus of non-linear viscoelastic spheres by microplate compression, and is applied to collagen microspheres fabricated with or without cells. During the measurement, a microsphere is placed on a rigid surface and is compressed by a calibrated flexible microplate gripped to a rigid end. A step increase in the displacement rate of the rigid end of the flexible microplate is introduced and the reduced elastic modulus of the microsphere is calculated from the deformation response of the microsphere, using an equation derived in this study. The reduced elastic modulus of collagen microspheres with and without mesenchymal stem cells measured by this method was 9.1 kPa and 132 Pa, respectively. © 2008 Biomedical Engineering Society.
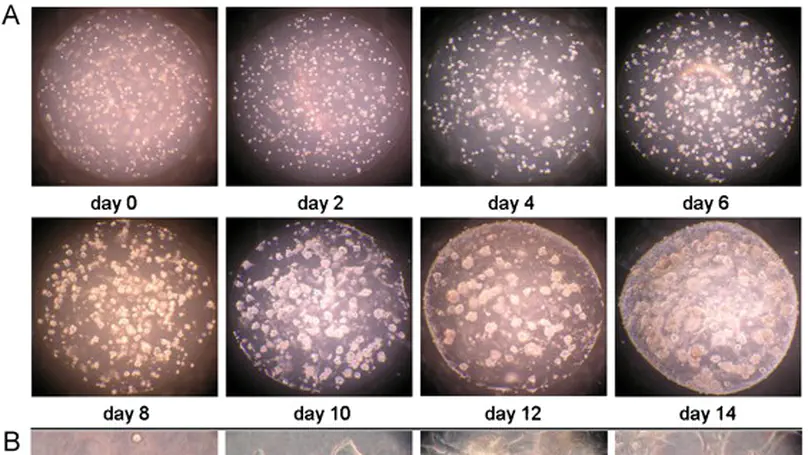
Mesenchymal stem cells (MSCs)-based therapy is a promising approach in regenerative medicine and tissue engineering. However, the outcomes of existing treatments have not been satisfactory owing to suboptimal localization to implantation site, poor viability, low engraftment efficacy and lack of functional remodeling of the delivered cells. Therefore, adopting an effective cell delivery modality is among the biggest technological challenges for successful clinical applications of MSC-based therapy. We developed a novel microencapsulation technique producing self-assembled collagen-MSC microspheres and demonstrated that these microspheres could serve as excellent cell delivery devices as they were stable, injectable and able to provide a protective, growth- and migration-supporting matrix to MSCs. We also showed that MSCs could preserve their stem cell nature upon microencapsulation and easily be localized with retained viability upon in vivo implantation. These microspheres present novel cell delivery devices with optimal biological and functional profile that may facilitate clinical applications of MSC-based therapy. © 2007 Elsevier Ltd. All rights reserved.
Mammalian cell culture technology has been used for decades in mass production of therapeutic proteins. However, unrestricted cell proliferation usually results in low-protein productivity. Controlled proliferation technologies such as metabolism intervention and genetic manipulation are therefore applied to enhance the productivity. Nevertheless, these strategies induced growth arrest with reduced viability and increased apoptosis. In this study, we report a new controlled proliferation technology by encapsulating human embryonic kidney (HEK) 293 cells over-expressing glial-derived neurotrophic factor (GDNF) in 3D collagen microspheres for extended culture. We investigated the viability, proliferation, cell cycle and GDNF productivity of HEK293 cells in microspheres as compared to monolayer culture. This system provides a physiologically relevant tissue-like environment for cells to grow and exerts proliferation control throughout the culture period without compromising the viability. A significant increase in the production rate of GDNF was found in the 3D microsphere system comparing with the monolayer culture. GDNF productivity was also significantly affected by the initial cell number and the serum concentration. The secreted GDNF was still bioactive as it induced neurite extension in PC12 cells. In summary, the 3D collagen microsphere system presents a cost-effective controlled growth technology for protein production in pharmaceutical manufacturing. © 2007 Elsevier Ltd. All rights reserved.