Chemical Modification of Collagen Improves Glycosaminoglycan Retention of Their Co-Precipitates
Abstract
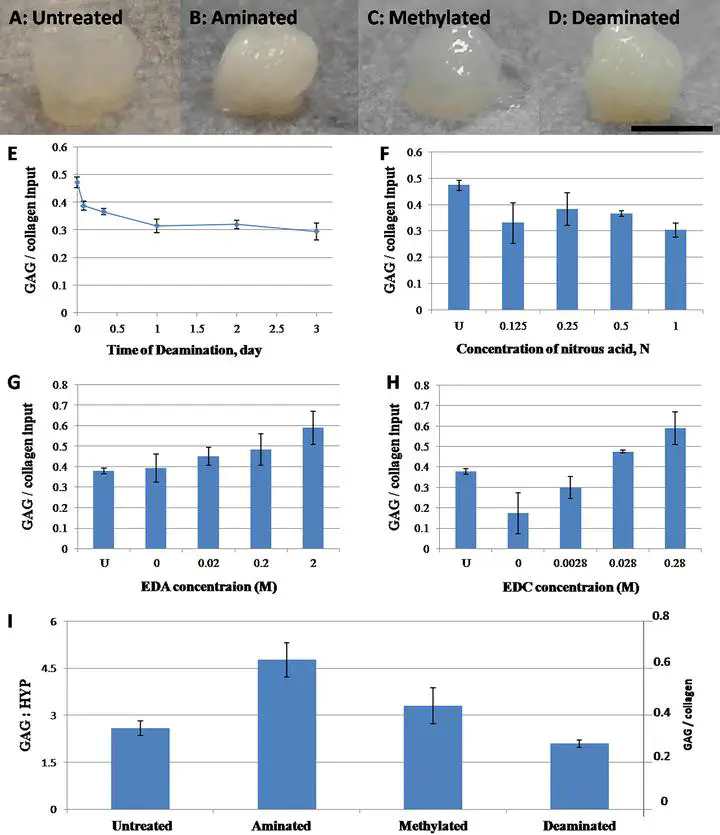
Being prevalent extracellular matrix components, collagen and glycosaminoglycan (GAG) are co-precipitated as scaffolds for tissue regeneration. However, the amount of GAG incorporated and its long-term retention present a persistent problem. In this study, chemical modifications, namely deamination, methylation and amination, were used to alter the net charge of collagen prior to fabrication of collagen-GAG co-precipitate. While most GAGs were lost in the untreated group and the deaminated group within 1 day, methylation and amination of collagen retained over 20% and 40% GAG after 6 days, respectively. Moreover, over 60% of GAG retention was achieved in the aminated group after cell seeding for 8 days. Furthermore, amination of collagen increased the GAG/hydroxyproline ratio in the co-precipitate to $>$4.5, approaching that of native nucleus pulposus. Ultrastructural analysis showed that the aminated group contains abundant granular substances resembling the extracellular matrix of native nucleus pulposus. Despite lower initial cell adhesion than untreated, all modified scaffolds promoted proliferation of human mesenchymal stem cells (hMSCs) and showed $>$95% cell viability at all time points. Cell morphology was distinct among the different groups, being round in the untreated control and methylated groups but elongated in deaminated and aminated groups. hMSCs adhered to scaffolds via collagen receptor integrin $α$2$β$1 in all groups, while all but the aminated group showed extensive expression of the general matrix receptor integrin $α$v. This work reports an effective method, namely amination of collagen, to improve GAG incorporation and retention in collagen-GAG co-precipitates, facilitating the fabrication of GAG-rich collagenous scaffold for intervertebral disc tissue engineering. © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.