Reconstitution of Bone-like Matrix in Osteogenically Differentiated Mesenchymal Stem Cell-Collagen Constructs: A Three-Dimensional in Vitro Model to Study Hematopoietic Stem Cell Niche
Abstract
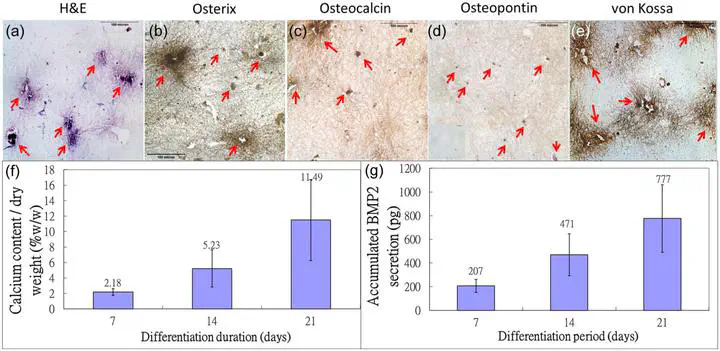
Mesenchymal stem/stromal cells (MSCs) and osteoblasts are important niche cells for hematopoietic stem cells (HSCs) in bone marrow osteoblastic niche. Here, we aim to partially reconstitute the bone marrow HSC niche in vitro using collagen microencapsulation for investigation of the interactions between HSCs and MSCs. Mouse MSCs (mMSCs) microencapsulated in collagen were osteogenically differentiated to derive a bone-like matrix consisting of osteocalcin, osteopontin, and calcium deposits and secreted bone morphogenic protein 2 (BMP2). Decellularized bone-like matrix was seeded with fluorescence-labeled human MSCs and HSCs. Comparing with pure collagen scaffold, significantly more HSCs and HSC-MSC pairs per unit area were found in the decellularized bone-like matrix. Moreover, incubation with excess neutralizing antibody of BMP2 resulted in a significantly higher number of HSC per unit area than that without in the decellularized matrix. This work suggests that the osteogenic differentiated MSC-collagen microsphere is a valuable three-dimensional in vitro model to elucidate cell-cell and cell-matrix interactions in HSC niche. © The Author(s) 2013.